Oncology
Oncology
Pipeline
We are a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic diseases. A covalent small molecule is a synthetic compound that forms a permanent bond to its target protein and offers a number of potential advantages over conventional non-covalent drugs, including greater target selectivity, lower drug exposure, and the ability to drive a deeper, more durable response.
We are utilizing our proprietary FUSION™ System to discover, design, and develop a pipeline of next-generation covalent-binding small molecule medicines designed to maximize clinical benefit for patients with various cancers and metabolic diseases, including diabetes. We aim to cure.
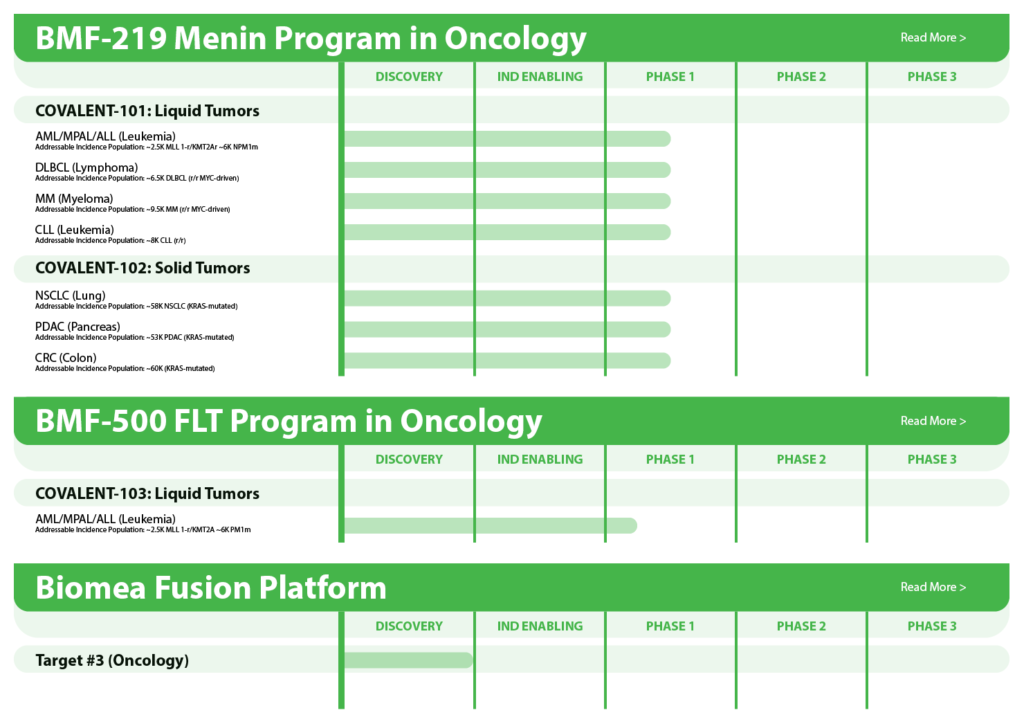
The following table summarizes our product candidate pipeline. We own full worldwide development and commercialization rights to all of our programs.
All our assets are in-house designed, developed, and wholly owned by Biomea Fusion Inc.
BMF-219 – Menin Inhibitor in Oncology
BMF-219 was discovered and developed in-house at Biomea using the company’s proprietary FUSION™ System. BMF-219 is a covalently binding inhibitor of menin, a protein known to play an essential role in oncogenic signaling in genetically defined leukemias as well as in diabetes. Preclinically, BMF-219 has demonstrated in well-established acute leukemia cell lines robust downregulation of key leukemogenic genes in addition to menin itself. Additionally, BMF-219 has shown anticancer activity in multiple in vitro, in vivo, and ex vivo models of acute leukemia, multiple myeloma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia. BMF-219 is currently being evaluated in clinical trials enrolling patients with specific menin-dependent mutations in liquid and solid tumors.
Clinical Development
In December 2023, Biomea reported the achievement of minimal residual disease negativity (MRD-neg) in one of the first complete responses (CRs) in AML. Within the total of 7 AML patients who received at least 2 cycles of therapy by the cutoff date, 2 CRs were observed with a mean time to response of 1.8 months. Pharmacodynamic data from a case study of an AML patient containing NUP98-NSD1 mutation further supports the proposed mechanism of action of BMF-219 as a menin inhibitor; in-line with preclinical models, BMF-219 downregulated key leukemogenic genes (e.g., HOXA9, MEIS1) as well as MEN1.
BMF-219 was generally well tolerated with no dose-limiting toxicities observed and without adverse event (AE)–related treatment discontinuations.
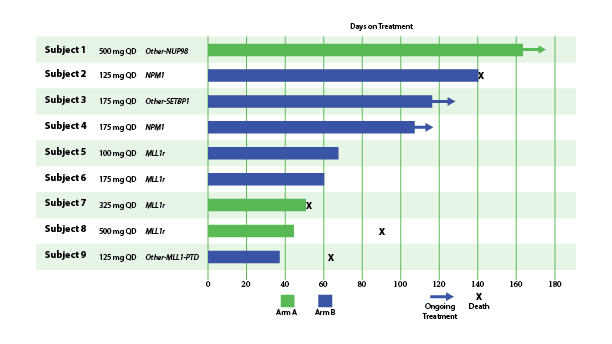
Fig. Early Signs of Efficacy: Time on Treatment (Lancet et al. ASH 2023)
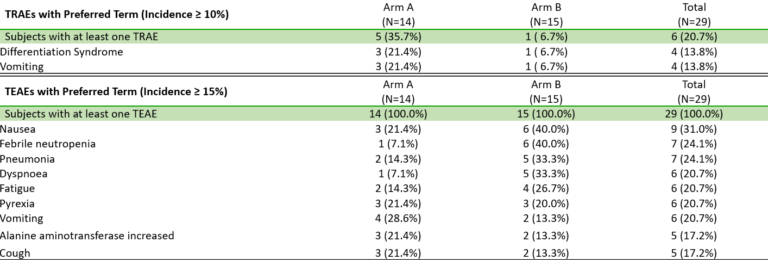
Fig. BMF-219 is Well Tolerated, Allowing for Continued Dose Escalation (Lancet et al. ASH 2023)
MLLr/mNPM1 Dependent AML/ALL
MLL1-r leukemias are characterized by MLL1 gene (encoded by the gene KM2TA) translocation abnormalities. These abnormalities result in the formation of fusion genes encoding fusion proteins comprised of MLL1 and a corresponding fusion partner domain. The interaction of these fusion proteins with menin drives the expression of downstream target genes such as HOXA9 and MEIS1, triggering leukemic cell proliferation.
Menin binds directly to the conserved N-terminus of MLL proteins, making it a promising target that could potentially be exploited consistently by a menin inhibitor therapeutic. Preventing the MLL proteins from binding to menin has been shown to abolish the oncogenic effects in vitro and in vivo.
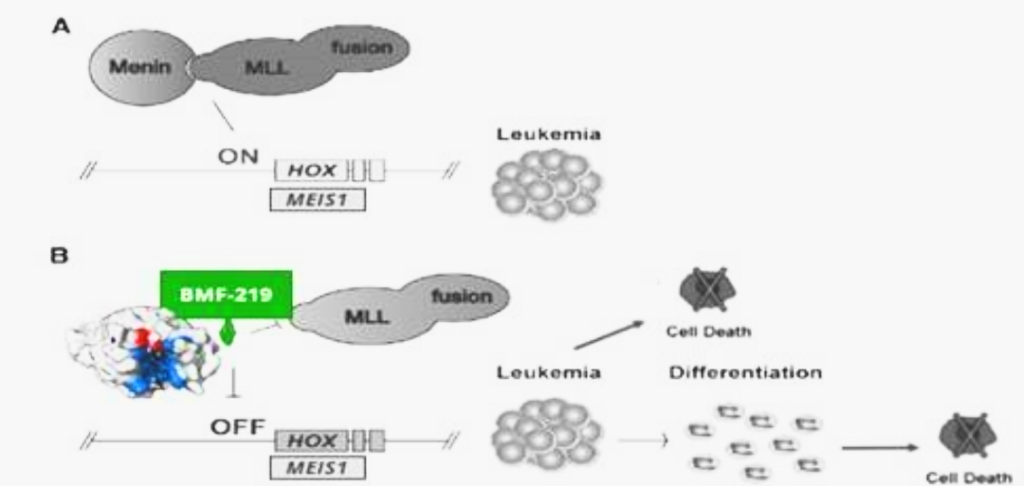
Fig. Depiction of leukemic pathway associated with aberrant menin-MLL fusion complex
Approximately 20,000 and 6,000 patients in the United States are diagnosed annually with AML and ALL, respectively. KMT2Ar leukemia has limited therapeutic options and affects approximately 5-10% of acute leukemias in adults and approximately 70% of acute leukemias in infants. In addition to KMT2A, MLL signaling in some forms of MLL wild-type (MLL-wt) AML have also been implicated, including those bearing independent oncogenic mutations in nucleophosmin (NPM1), a molecular chaperone, and DNA-methyltransferase 3A (DNMT3A), a methyl transferase. These subpopulations together represent approximately 35-40% of AML cases.
A perhaps more dire area of unmet need is relapsed/refractory AML. Despite evolving insights into the pathogenesis of AML, over 11,000 patients with AML die each year from the disease in the United States. Relapse is the most common cause of treatment failure. The 5-year overall survival (OS) for adult patients with AML after disease relapse is only approximately 10%. Given the involvement of KMT2A and NPM1 in a high percentage of acute leukemias, and the poor clinical outcomes provided by available treatments, we believe a new treatment that can inhibit the function of both targets by disrupting or preventing interactions with menin could address this unmet need.
MYC Driven Liquid Tumors (MM, DLBCL, CLL)
MYC is a transcription factor that is implicated in oncogenesis and typically regulates genes associated with cellular proliferation, differentiation, and apoptosis. In fact, MYC is constitutively and aberrantly expressed in over 70% of human cancers. MYC dysregulation can occur due to a number of different factors, including RAS activation. MYC appears to play a key role in the functioning of many cancer cells, including Diffuse large B cell lymphoma (DLBCL), Multiple Myeloma (MM), Chronic lymphocytic leukemia (CLL), and KRAS solid tumors (Colorectal, Pancreatic, and Lung). MYC is aberrantly expressed in relapsed / refractory DLBCL and MM and is a downstream effector of KRAS mutant tumors.
Menin has been shown to play an essential role in the MYC transcriptional complex, which leads to menin-mediated enhancement of MYC target gene expression in cancer cells.
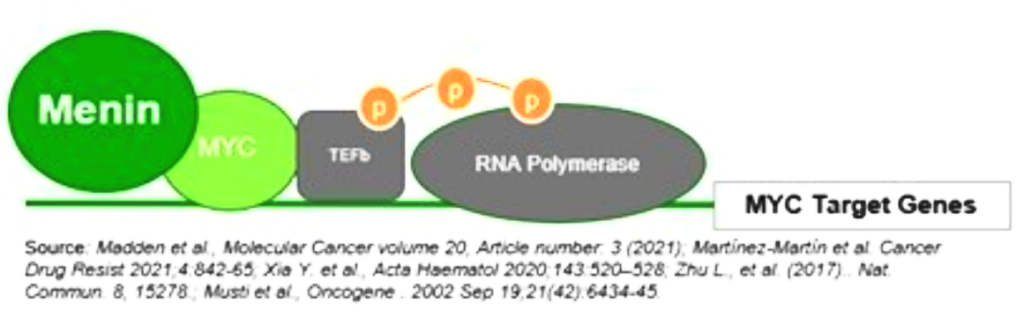
Fig. The role of menin in the MYC transcriptional complex, facilitating the expression of MYC target genes
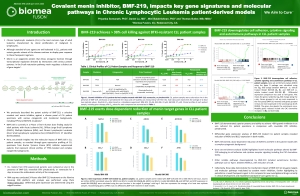
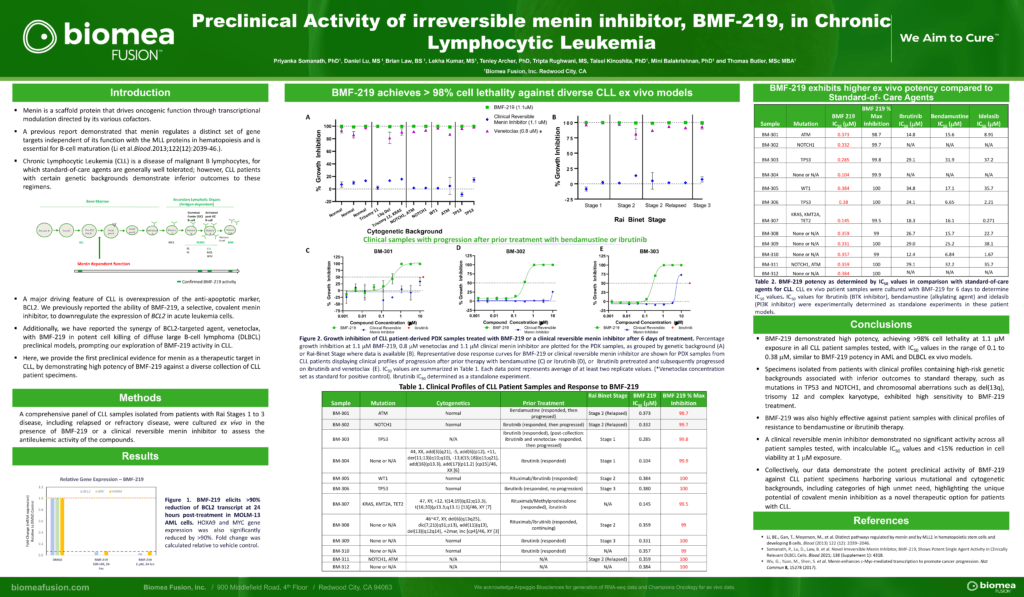
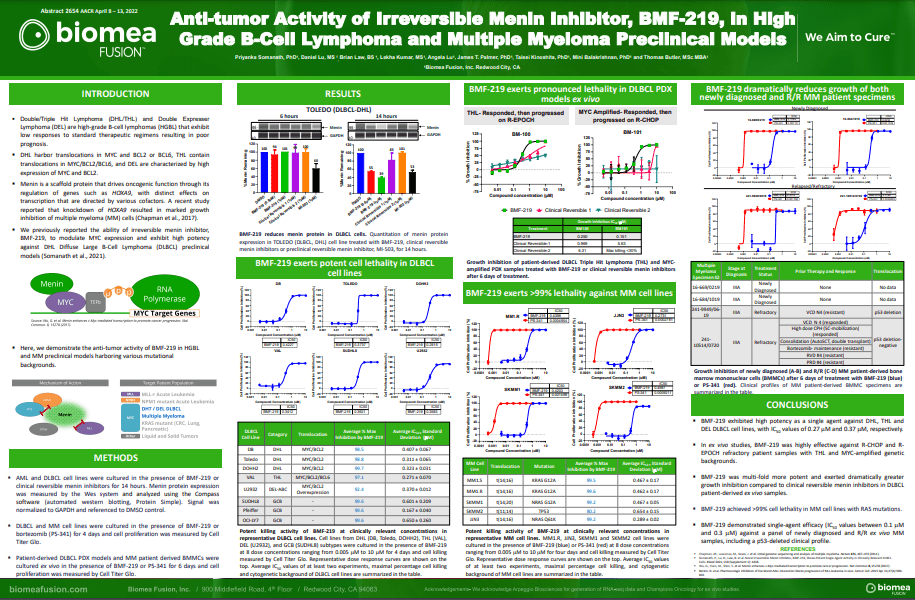
BMF-219 demonstrated robust anti-tumor activity and mechanistic evidence for novel inhibition of the menin protein in preclinical models of DLBCL and multiple myeloma MM. BMF-219 has also shown potency across ex vivo CLL tumor models with varying cytogenetic risk profiles and Rai stages, indicating broad activity with over 98% cell lethality in these models.
MM is a cancer of plasma cells, which make antibodies and are mainly located in the bone marrow. Approximately 35,000 people in the U.S. are diagnosed with MM each year and the 5-year relative survival rate is ~56% (Source: NCI SEER Data). While many therapeutic options are available to patients, a subset of highly treatment-refractory patients exists. In these patients, overall survival is as low as 6 months. Additionally, it is estimated that more than 60% of MM patients have menin-dependent genetic drivers (MYC addicted or driven) and that these drivers are more common in the relapsed or refractory setting.
DLBCL is the most common subtype of Non-Hodgkin Lymphoma. Every year, approximately ~18,000 people in the U.S. are diagnosed with DLBCL (Source: NCI SEER Data). Following initial treatment with standard chemotherapy, approximately 70% of patients have a complete response and approximately 50% of patients are cured. There is a substantial unmet need for patients with relapsed or refractory DLBCL as median overall survival is between 6-7 months in this group. Double Hit Lymphomas (DHL), Triple Hit Lymphomas (THL), and Double Expressor Lymphomas (DEL) are high grade B-cell lymphomas (HGBLs) that have high MYC and BCL2 dependency. Based on their aggressive nature, DHL, THL, and DEL represent a large portion of the relapsed or refractory DLBCL population.
CLL is a chronic leukemia that progresses relatively slowly and typically impacts older adults. In the United States, approximately 20,000 patients are diagnosed with CLL each year. CLL is a disease of malignant B lymphocytes, for which standard-of-care agents are generally well tolerated; however, CLL patients with certain genetic backgrounds demonstrate inferior outcomes to these regimens. While the existing treatment options produce 5-year survival outcomes greater than 87%, there is an unmet need for patients that have high- or medium-risk cytogenetic profiles and those that are relapsed or refractory to existing treatments.
–
MYC Driven KRAS Solid Tumors (Lung, Colon, Pancreatic Cancer)
BMF-219 is the first menin inhibitor to enter clinical trials for the treatment of solid tumors. A targeted pan-KRAS inhibitor has the potential to treat 25-35% of NSCLC, 35-45% of CRC, and approximately 90% of PDAC patients. In a series of preclinical studies, BMF-219 showed strong and highly specific pan-KRAS anti-cancer activity as a single agent across KRAS G12C, G12D, G12V and G13D mutations, including in NSCLC, CRC, and the most prevalent type of pancreatic cancer, PDAC.
Non-Small Cell Lung Cancer (NSCLC) is the most common form of lung cancer, representing ~84% of all lung cancer cases or approximately 200,000 cases in the U.S. each year (Source: NCI SEER Data). Additionally, the five-year survival rate of NSCLC is ~25%. KRAS is a key node in the RAS signaling pathway and the most frequent oncogene in NSCLC, occurring in ~30% of patients with NSCLC.
Pancreatic ductal adenocarcinoma (PDAC) is a relatively rare form of cancer in the U.S., representing approximately 60,000 cases in the U.S. each year (Source: NCI SEER Data). It is an aggressive cancer with a very low five-year survival rate of ~11%, indicating that there is a large unmet need. It is rarely diagnosed early, contributing to the low survival rate. Among patients with pancreatic cancer, RAS mutations (including KRAS) occur in up to approximately 98% of patients.
Colorectal cancer (CRC) is the fourth most common form of cancer in the U.S., representing approximately 150,000 cases in the U.S. each year (Source: NCI SEER Data). These cancers start in the rectum or the colon and can be diagnosed/identified early, even potentially as noncancerous polyps. The five-year survival rate of CRC is approximately 65%. Among other mutations, KRAS mutations occur in approximately 50% of patients with CRC.
BMF-500 - FLT3 Inhibitor in AML
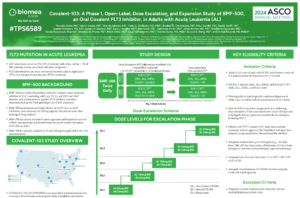
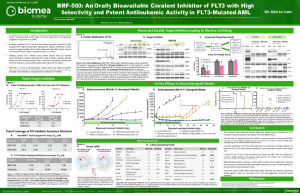
BMF-500 is an investigational, novel, orally bioavailable, highly potent and selective covalent small molecule inhibitor of fms-like tyrosine kinase 3 (FLT3). Biomea has continued advancing BMF-500 toward the clinic, with an investigational new drug (IND) application cleared in 1H2023 and a Phase 1 study of BMF-500 (COVALENT-103; NCT05918692) initiated and first patient dosed in 2H2023.
BMF-500 was discovered and developed in-house at Biomea using the company’s proprietary FUSION™ System and has demonstrated best-of-class potential based on extensive preclinical studies. The kinase profile of BMF-500 showed high target selectivity, suggesting the potential for minimal off-target liabilities. BMF-500 was designed to have a therapeutic profile to allow for combinations with standard of care and/or novel targeted agents like BMF-219.
FLT3 is a receptor tyrosine kinase (RTK) that plays a central role in the survival, proliferation, and differentiation of immature blood cells. Notably, FLT3 gene mutations are common in patients with AML and are associated with a poor prognosis. Nearly 30% of AML patients have a FLT3 mutation, representing more than 6,000 incident patients in the United States each year. While FLT3-specific and pan-tyrosine kinase inhibitors are approved by the FDA across various lines of therapy in AML, these agents have produced relatively low rates of durable responses, and overall survival remains an unmet need.
Previous data presented at medical conferences showed BMF-500’s picomolar affinity to activating FLT3 mutations, including FLT3-ITD and various tyrosine kinase domain (TKD) mutations (Law et al. ASH 2022). BMF-500 demonstrated multi-fold higher potency and increased cytotoxicity than commercially available non-covalent FLT3 inhibitor gilteritinib. Further data also exhibited the potential utility of combination strategies to achieve higher antileukemic cell killing with reduced concentrations of BMF-500 and BMF-219 (Law et al. AACR 2023). These data provide preclinical evidence for combining pathway-specific inhibitors as a promising therapeutic strategy for further investigation in acute leukemia.
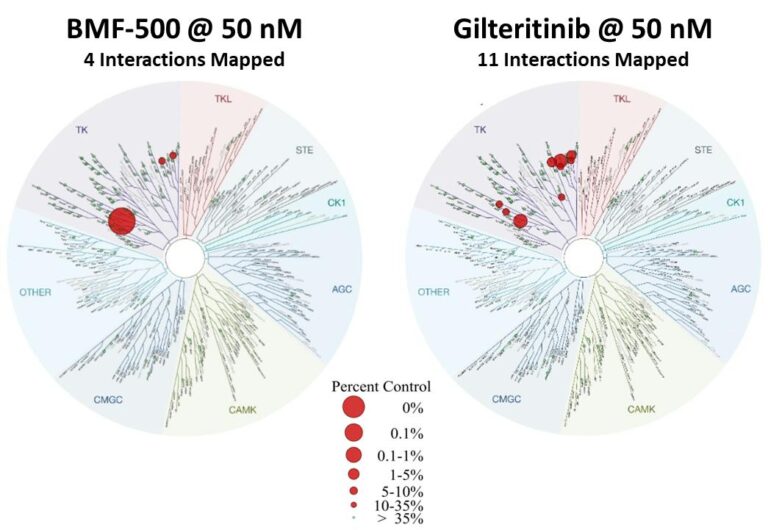
Fig. BMF-500 is highly selective to FLT3 (Law et al. ASH 2022).
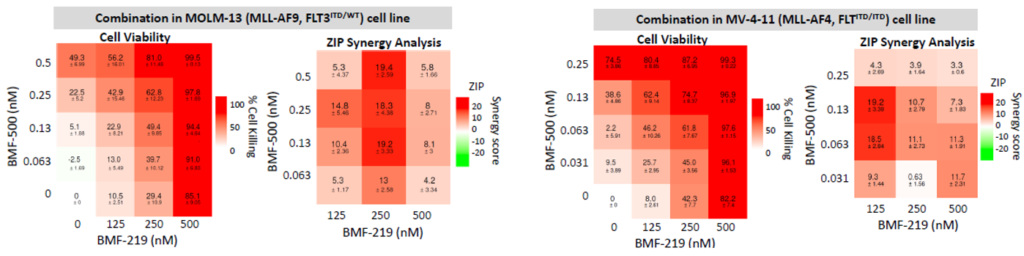
Fig. BMF-219 and BMF-500 in Combination Induced Higher Cell Killing at Lower Single Agent Concentrations (Law et al., AACR 2023)
Abstracts
- Farhad Ravandi, Ashwin Kishtagari, Hetty E Carraway, Gary J. Schiller, Steve Morris, Alexandru Cacovean, Bhagyashree (Kelshikar) Yadav, Thomas Butler, Jeffrey E. Lancet; COVALENT-101: A phase 1 study of BMF-219, a novel oral irreversible menin inhibitor, in patients with relapsed/refractory (R/R) acute leukemia (AL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma (MM). J Clin Oncol 40, 2022 (suppl 16; abstr TPS7064)
https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.TPS7064
- Priyanka Somanath, Daniel Lu, Brian Law, Tenley Archer, Tripta Rughwani, Lekha Kumar, Taisei Kinoshita, Mini Balakrishnan, Thomas Butler; Preclinical activity of irreversible Menin inhibitor, BMF-219, in chronic lymphocytic leukemia. J Clin Oncol 40, 2022 (suppl 16; abstr 7541)
https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.7541
- Farhad Ravandi-Kashani, Ashwin Kishtagari, Hetty Carraway, Emily Curran, Gary Schiller, Alex Cacovean, Bhagyashree Yadav, Thomas Butler, Jeffrey Lancet. A phase 1 study of BMF-219, a novel oral irreversible menin inhibitor, as single-agent in patients with relapsed/refractory (R/R) acute leukemia (AL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma (MM) In AACR; April 8-13, 2022; New Orleans, LA https://www.abstractsonline.com/pp8/#!/10517/presentation/20306
- Brian Law, Daniel Lu, Priyanka Somanath, James T. Palmer, Taisei Kinoshita, Thomas Butler. Irreversible menin inhibitor, BMF-219, inhibits the growth of KRAS-mutated solid tumors. In AACR; April 8-13, 2022; New Orleans, LA https://www.abstractsonline.com/pp8/#!/10517/presentation/15102
- Priyanka Somanath, Daniel Lu, Brian Law, James T. Palmer, Taisei Kinoshita, Mini Balakrishnan, Thomas Butler. Anti-tumor activity of irreversible menin inhibitor, BMF-219, in High-Grade B-Cell Lymphoma and Multiple Myeloma preclinical models. In AACR; April 8-13, 2022; New Orleans, LA https://www.abstractsonline.com/pp8/#!/10517/presentation/15520
- Priyanka Somanath, Daniel Lu, Brian Law, Tenley C. Archer, Alexandru Cacovean, James T. Palmer, Taisei Kinoshita, Thomas Butler; Novel Irreversible Menin Inhibitor, BMF-219, Shows Potent Single Agent Activity in Clinically Relevant DLBCL Cells. Blood 2021; 138 (Supplement 1): 4318. https://doi.org/10.1182/blood-2021-148045