PIPELINE &
INNOVATION
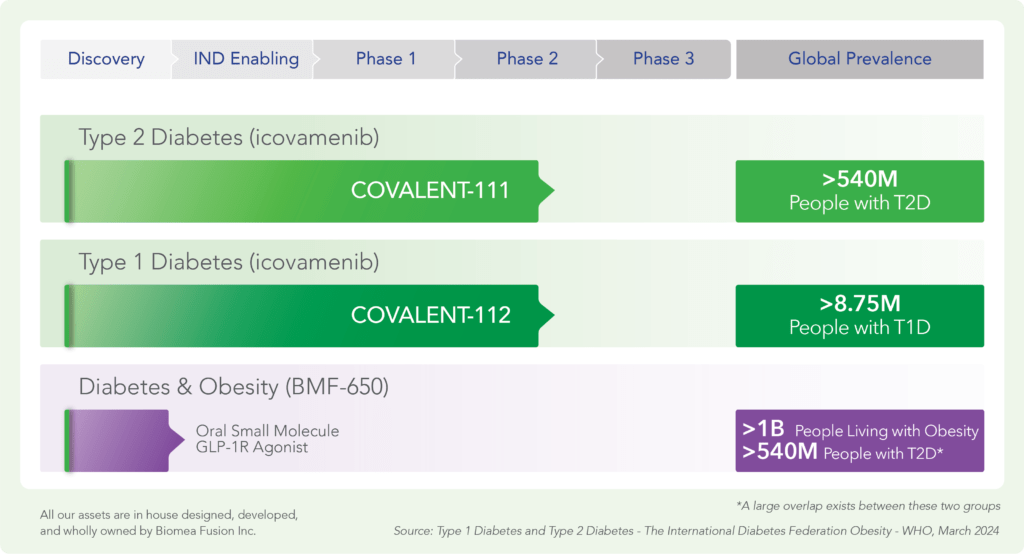
Pipeline
Our Science
Biomea Fusion is creating a new generation of small molecule covalent inhibitors that target genetically defined diseases with high unmet need. These diseases all have biomarker-identifiable patient populations that will be targeted via in silico designed small molecules with new mechanisms of action that likely become best in class. The in-silico and preclinical work is designed to produce an optimized product profile of high potency with benign safety (“Clean Drugs”). We believe that the product profile (best in class potency and safety) and genetically defined disease drivers putatively reduce the technical risk of each project. Covalent drugs provide a new path to address unmet medical needs and through their innovative mechanism of action will have significant clinical and financial value, if successfully developed.
Key Advantages of Covalent Drugs
Since the discovery of aspirin in 1899, covalent drugs have been known to offer a number of potential safety, tolerability and efficacy advantages over conventional non-covalent drugs through multiple mechanisms, including:
- High selectivity: Covalent drugs have the potential to confer high selectivity to a target by interacting with the unique surrounding structural elements of the protein and establishing a covalent bond to a key residue in the binding site. Leveraging non-covalent and covalent interactions can lead to greater selectivity versus non-covalent compounds, which rely solely on non-covalent binding. This has the potential to reduce the likelihood of non-specific, off-target interactions that often lead to safety and tolerability concerns.
- Deep inactivation of target: Upon binding, a covalent inhibitor not only causes inactivation of the target, but may also result in the elimination of the target through normal cellular degradation processes. The diseased cell then either undergoes rapid apoptosis or differentiation into a normal, mature cell. Such transformation has the potential to provide the patient with a durable, lasting benefit.
- Greater therapeutic window: Covalent inhibitors are designed to create a permanent bond with high affinity and long residence time. Unlike conventional non-covalent drugs, which typically need to be present continuously to provide benefit, covalent drugs have the potential to maintain their effect in the absence of sustained drug exposure. The permanent inhibition of target function upon covalent binding essentially uncouples PD (drug effects) from PK (drug exposure), as target inhibition persists after the drug has been cleared from the system. This property of covalent drugs can potentially lead to lower drug doses and less frequent dosing regimens versus non-covalent approaches. Figure 1 below highlights the potential PD and PK benefits of an FDA-approved covalent BTK inhibitor (ibrutinib). In particular, the model shows the ability of a covalent inhibitor to quickly achieve maximum target engagement and sustain inhibition while the drug is rapidly cleared from the body, which further reduces the potential for off-target interactions and non-mechanism-based toxicities. These features have enabled ibrutinib to achieve sustained efficacy with lower exposure and a favorable safety profile.
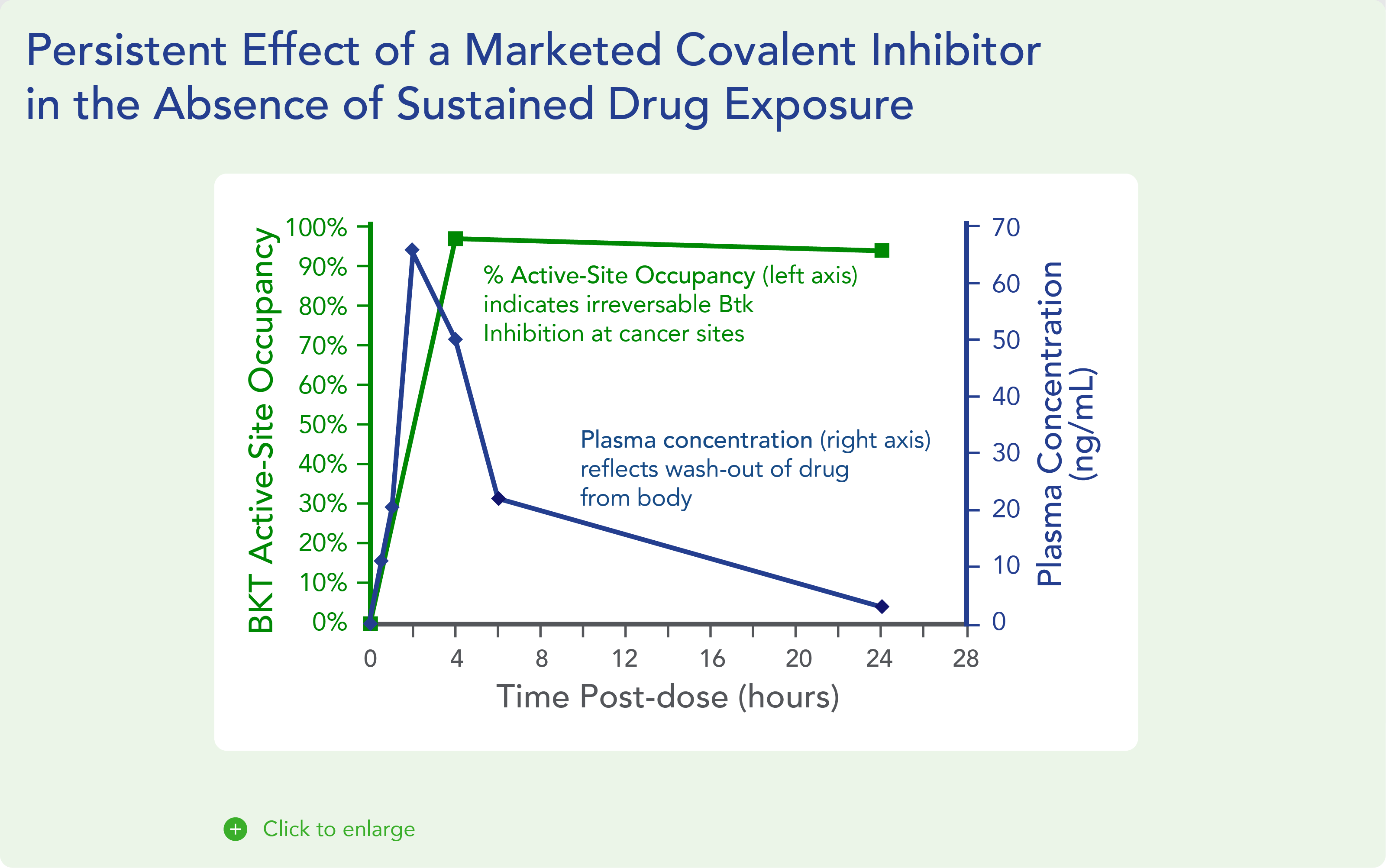
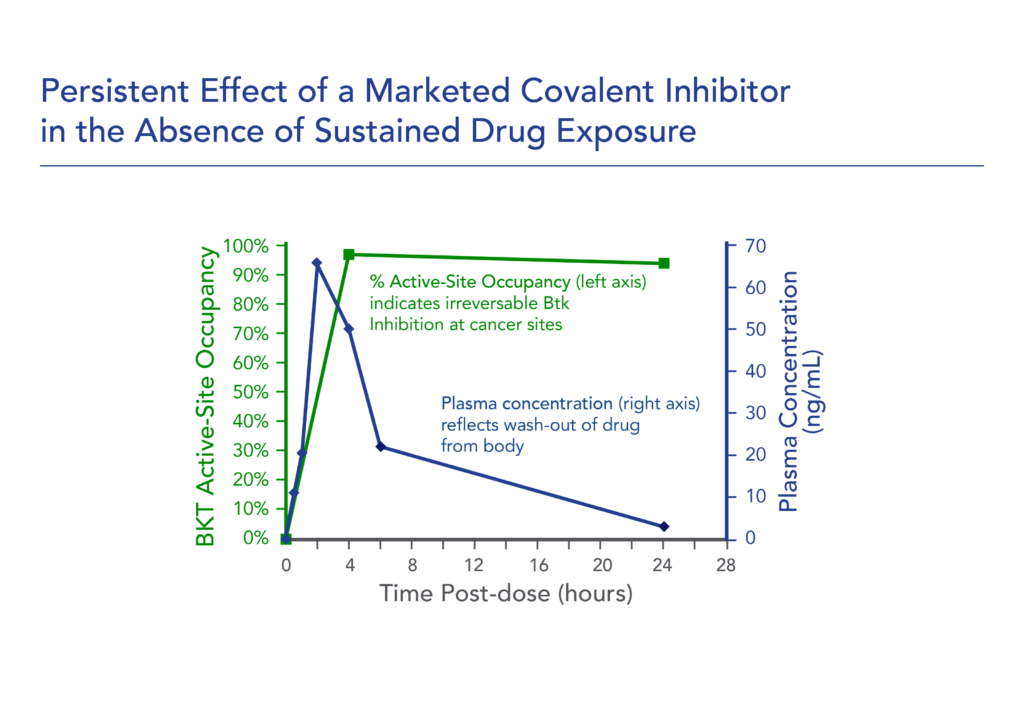
Fig. 1. Persistent effect of a marketed covalent inhibitor in the absence of sustained drug exposure
Beyond aspirin and ibrutinib, a number of covalent inhibitors have been developed to improve patient outcomes, including sofosbuvir (marketed as SOVALDI for hepatitis C virus), tenofovir (marketed as VIREAD for hepatitis B virus), osimertinib (marketed as TAGRISSO for NSCLC), and bortezomib (marketed as VELCADE for multiple myeloma and mantle cell lymphoma).
Challenges in Developing Covalent Drugs
Despite the potential advantages of covalent drugs, the majority of approved drugs are non-covalent binders. The inherent challenges in creating covalent drugs presents significant barriers to entry to discover and develop these molecules. The key challenges in developing covalent drugs include:
- Complexity: The discovery and development of covalent drugs requires significant structural knowledge and medicinal chemistry capabilities, including the ability to construct complex novel chemical scaffolds. In addition, not all disease-causing proteins have the properties necessary for the application of covalent binding. While advancements in structural knowledge of the proteome provide greater opportunity to identify potential targets for covalent binding, the lack of specialized medicinal chemistry expertise needed to leverage this knowledge has impeded the development of covalent drugs.
- Safety and toxicity: While the covalent binding modality can provide a high degree of selectivity, poorly conceived molecules with promiscuous binding profiles can result in significant off-target interactions and safety concerns. Given this significant and long-standing challenge, drug developers have historically been discouraged from pursuing covalent binders.
At Biomea, we are able to leverage the significant expertise, foundational knowledge, and capabilities that our management team first acquired while developing ibrutinib and that we have expanded and refined over the last three years to create our FUSION™ System discovery platform.
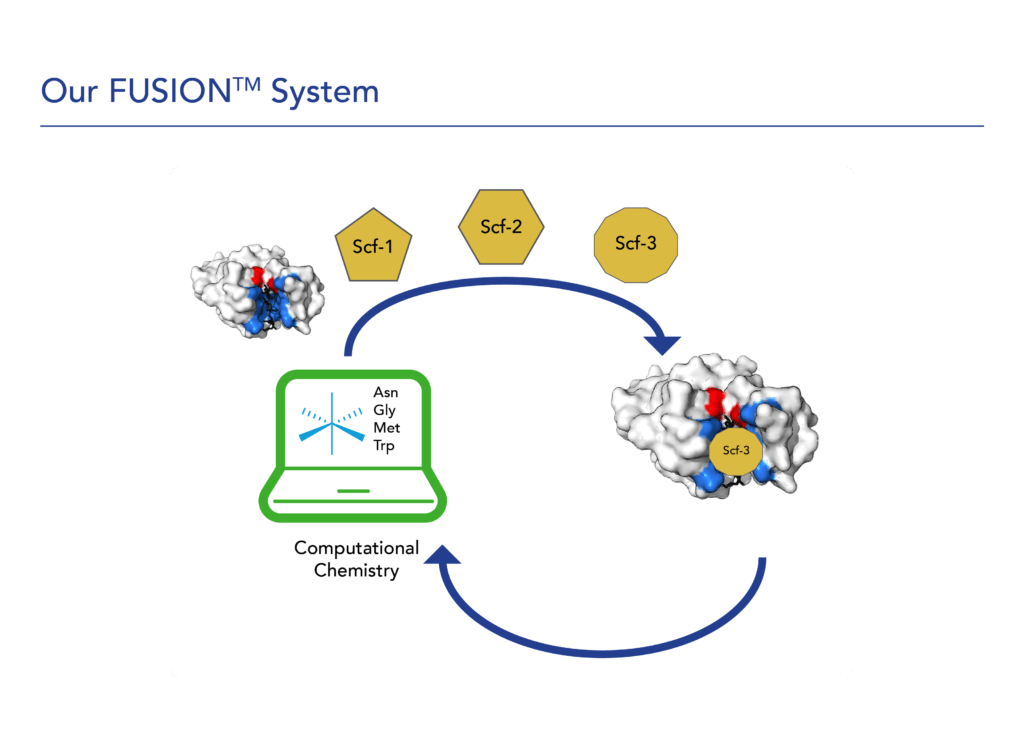
Our FUSION™ System
We believe that covalent small molecules have the potential to address the key limitations of existing non-covalent therapeutics and treat diseases where targeted therapies are not yet approved. Leveraging our extensive experience developing covalent drugs and covalent binding chemistry expertise, we built our proprietary FUSION™ System to enable the design and development of novel covalent, small molecule therapies against high-value genetic drivers of cancer. Our FUSION™ System discovery platform encompasses:
- Target selection: We use our expertise in structural biology and covalent binding chemistry to identify both validated and novel targets that have demonstrable and specific impact on disease and have particular structural characteristics amenable to direct intervention with a covalent binder.
- Scaffold creation: We create novel chemical scaffolds using a computational platform to exploit the unique structural elements of a specific target protein. We then screen these scaffolds with in-house technologies to select the optimal candidates for further construction and design. This evaluation process is intended to increase the probability of advancing multiple targeted compounds through the discovery process and into the clinic.
- Molecule optimization: Using our proprietary suite of computational technologies, assays, analytical approaches, chemistry, and know-how, we strive to maximize the selectivity, potency, and safety of our covalent small molecules.
We aim to leverage our capabilities and platform to establish ourselves as a leader in developing covalent small molecules in order to maximize the depth and durability of clinical benefit for patients with various cancers.
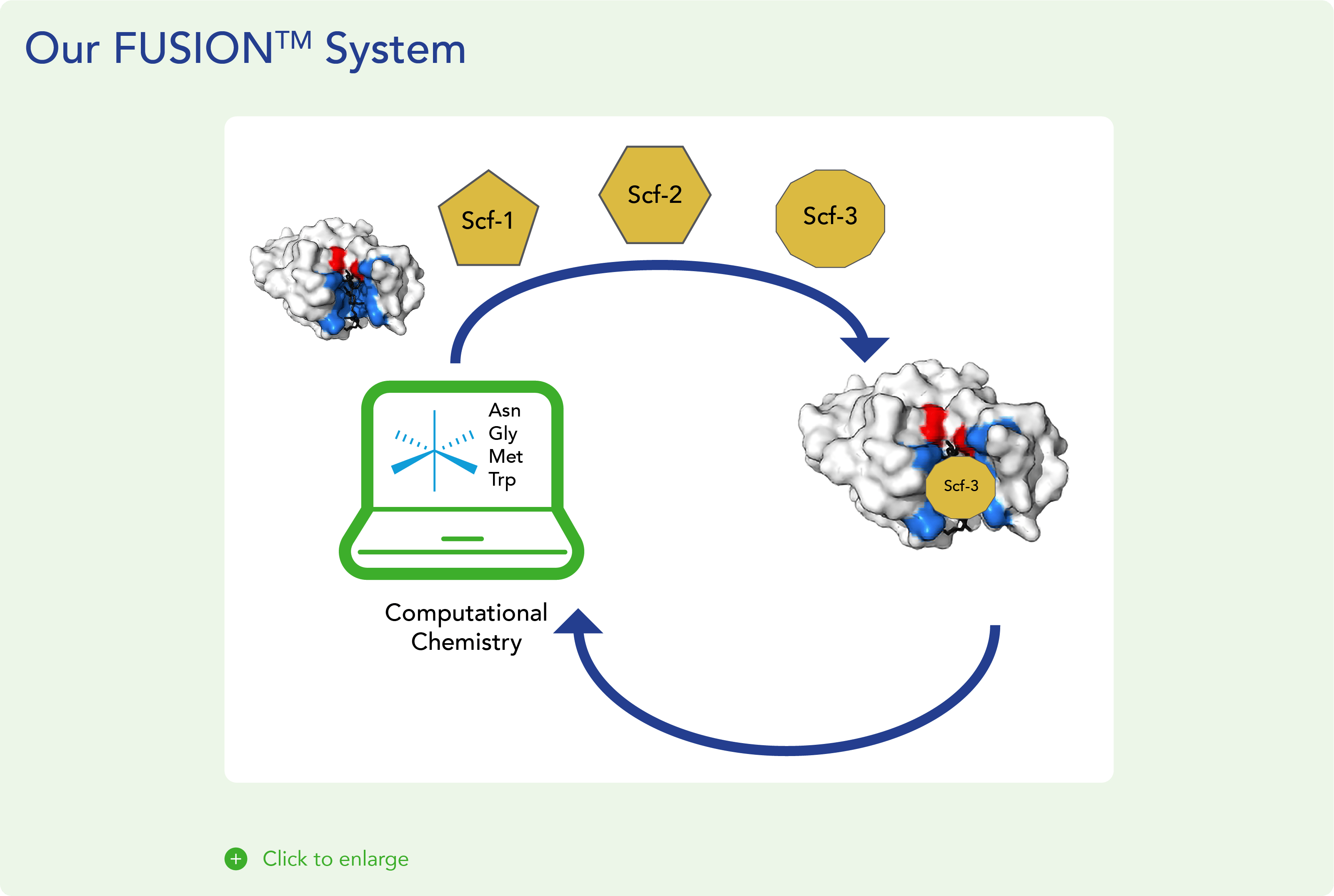
We leverage unique scaffold designs based on crystal structure analysis with our team’s deep experience in covalent small molecule drug development. The Biomea Fusion team members were involved in creating some of the most exciting compounds in commercial use today. Top of the list is PCI-32765, a covalent inhibitor of BTK, which achieves precise target inhibition with a benign safety profile. This compound is commercially available as IMBRUVICA, with over $6B of yearly sales and a label that shows overall survival benefits.
We also apply rigorous safety assessments as an integral part of our compound selection processes. We use standard receptor screening tools as well as in-vivo single and ascending dosages with multiple schedules to ensure our compounds are safe in all dosing and usage circumstances. We strive to provide patient-friendly and targeted solutions to address complex diseases. Particularly in oncology, many existing therapies often use aggressive toxins in the hope of controlling the spread of the disease which leads to a myriad of side effects. We aim to laser focus on the target and to do so with incredibly effective compounds that have been preclinically tested and shown to have relatively benign safety profiles.
