PIPELINE
Overview
We are a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic diseases. A covalent small molecule is a synthetic compound that forms a permanent bond to its target protein and offers a number of potential advantages over conventional non-covalent drugs, including greater target selectivity, lower drug exposure, and the ability to drive a deeper, more durable response.
We are utilizing our proprietary FUSION™ System to discover, design and develop a pipeline of next-generation covalent-binding small molecule medicines designed to maximize clinical benefit for patients with various cancers and metabolic diseases, including diabetes. We aim to cure.
The following table summarizes our product candidate pipeline. We own full worldwide development and commercialization rights to all of our programs.
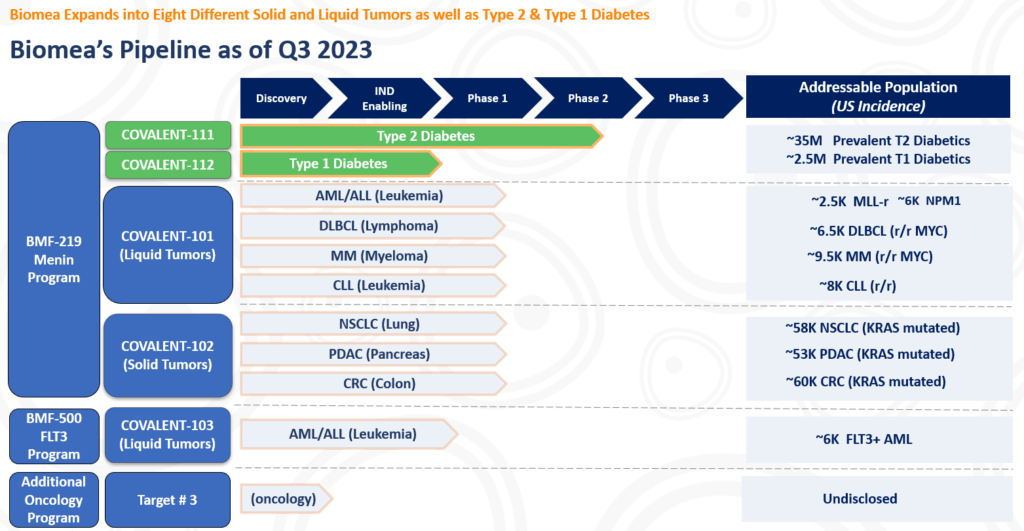
All our assets are in house designed, developed, and wholly owned by Biomea Fusion Inc.
BMF-219 in patients with Acute Myeloid Leukemia (AML), Multiple Myeloma (MM), Diffuse Large B-Cell Lymphoma (DLBCL), and Chronic Lymphocytic Leukemia (CLL)
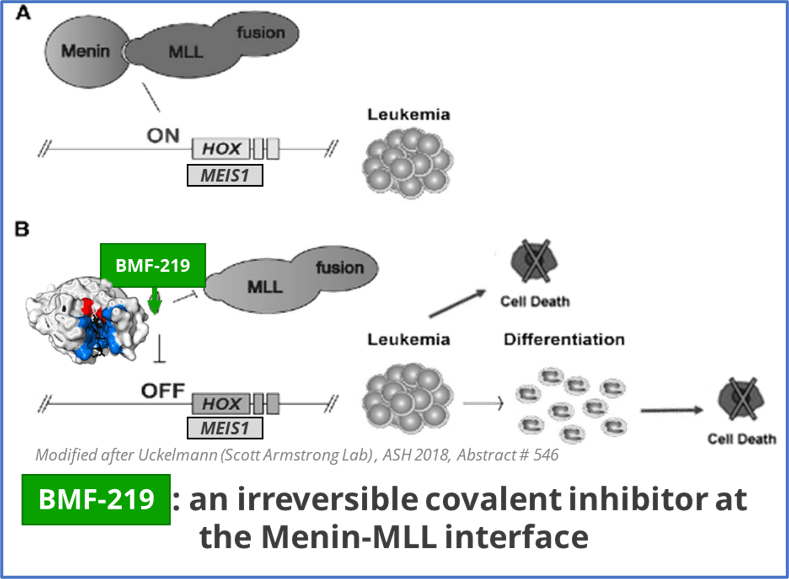
BMF-219 is an oral investigational covalent menin inhibitor. Data suggests that optimized covalent inhibitors can provide deeper inhibition while being better tolerated than some conventional reversible inhibitors.
BMF-219 is being developed for genetically defined AML, ALL, DLBCL, MM and CLL patients.
BMF-219 blocks the interaction of menin and MLL (AML, ALL), and limits the activity and/or expression of NPM1, MYC, HOX, and MEIS1, all known drivers of oncogenic proliferation and survival.
Mixed lineage leukemia (MLL) harboring rearrangements (MLLr) in acute leukemias confer poor outcomes. MLLr when bound to menin, drives oncogenic activity including the increase in HOXA expression. High expression of HOXA genes correlates with poor clinical outcome in acute myeloid leukemias. HOXA overexpression is observed in >70% of human AML cases and ~10% of ALL cases.
Similarly, MYC activity is heavily dependent on its interaction with menin, which BMF-219 can strongly inhibit (in preclinical models). MYC addiction increases with stage and line of therapy in MM and DLBCL. ~20-50% newly diagnosed MM patients and ~50-70% of advanced r/r MM patients have MYC dysregulation. While Double and Triple Hit and Double expressors (BCL2 and MYC overexpression) DLBCL impacts ~40% of patients.
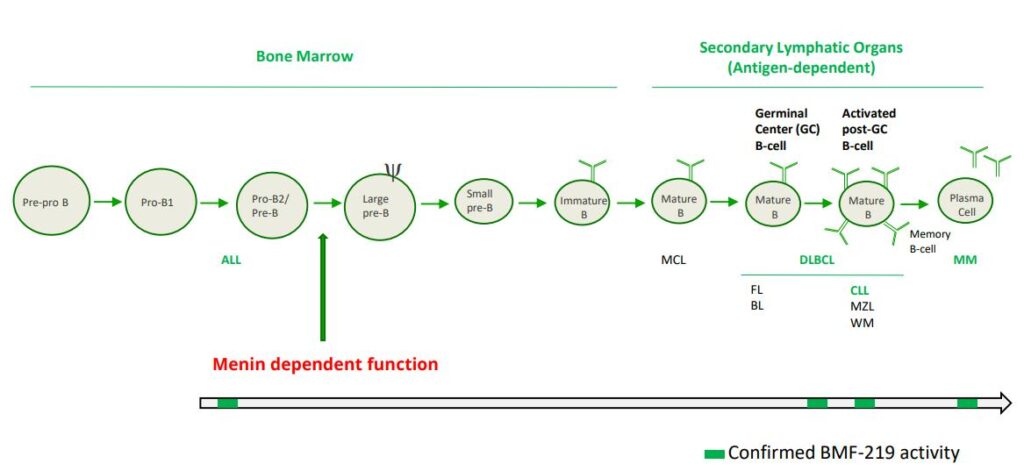
In addition, a previous report demonstrated that menin regulates a distinct set of gene targets independent of its function with the MLL proteins in hematopoiesis and is essential for B-cell maturation (Li et al.Blood.2013;122(12):2039-46.). CLL is a disease of malignant B lymphocytes. A major driving feature of CLL is overexpression of the anti-apoptotic marker, BCL2, which BMF-219 can downregulate shown in preclinical models.
Menin Dependent ALL and AML
Approximately 20,000 and 6,000 patients in the United States are diagnosed annually with AML and ALL, respectively. MLL-r leukemia has limited therapeutic options and affects approximately 10% of acute leukemias in adults and approximately 70% of acute leukemias in infants. In addition to MLL-r, MLL signaling in some forms of MLL wild-type (MLL-wt) AML have also been implicated, including those bearing independent oncogenic mutations in nucleophosmin (NPM1), a molecular chaperone, and DNA-methyltransferase 3A (DNMT3A), a methyl transferase. These subpopulations together represent approximately 45% of AML cases.
Patients with MLL rearrangements often suffer from failure of induction therapy or disease relapse, resulting in poor clinical outcomes. In pediatric AML, the five-year event-free survival rate on average is 44% but ranges between 11% and 92% depending on the MLL-translocation subtypes. In ALL, the five-year survival rate for people aged 20 and older is approximately 37% and for people under the age of 20 it is approximately 89%. However, pediatric MLL-r ALL patients fare much worse, with four-year survival rates as low as 10%, compared to 64% for those without MLL rearrangements.
A perhaps more dire area of unmet need is relapsed/refractory AML. Despite evolving insights into the pathogenesis of AML, over 11,000 patients with AML die each year from the disease in the United States. Relapse is the most common cause of treatment failure. The five-year overall survival (OS) for adult patients with AML after disease relapse is only approximately 10%. Furthermore, a published study showed that approximately 20% of patients demonstrated primary induction failure adding even more patients to this refractory category. Currently, allogeneic hematopoietic cell transplantation (HCT) is considered to be the only reliable option with curative potential, with OS estimated between 15% to 25% three to five years post-transplant. To improve overall quality of life for patients, physicians are favoring oral targeted agents and strategies that avoid intensive chemotherapy and prolonged inpatient admissions. Key in this effort is a focus on molecular testing to identify the potential for targeted therapies.
Given the involvement of MLL and NPM1 in a high percentage of acute leukemias, and the poor clinical outcomes provided by available treatments, we believe a new treatment that can inhibit the function of both targets by disrupting or preventing interactions with menin could address this unmet need.
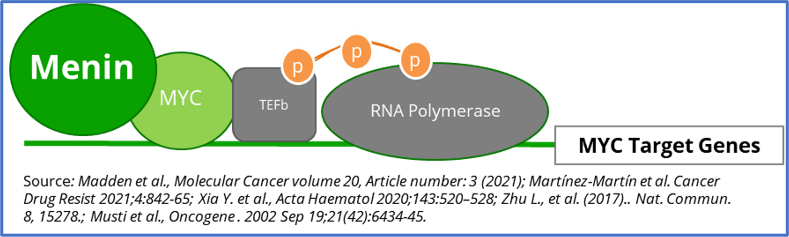
Menin & MYC – Diffuse Large B-Cell Lymphoma (DLBCL), Multiple Myeloma (MM), and KRAS Solid Tumors (Lung, Pancreatic, and Colorectal)
MYC is a transcription factor that is implicated in oncogenesis and typically regulates genes associated with cellular proliferation, differentiation, and apoptosis. In fact, MYC is constitutively and aberrantly expressed in over 70% of human cancers. As shown in the figure below, MYC dysregulation can occur due to a number of different factors, including RAS activation. MYC appears to play a key role in the functioning of many cancer cells, including DLBCL, MM, and KRAS solid tumors (Colorectal, Pancreatic, and Lung). MYC is aberrantly expressed in relapsed / refractory DLBCL and MM and is a downstream effector of KRAS mutant tumors. As shown in the prior figure, Menin has been shown to play an essential role in the MYC transcriptional complex, which leads to menin-mediated enhancement of MYC target gene expression in cancer cells.
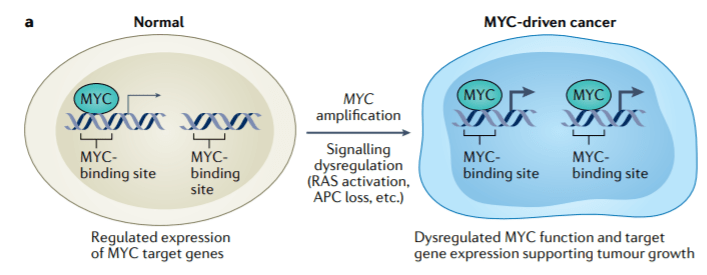
Source: Lourenco, C., Resetca, D., Redel, C., Lin, P., MacDonald, A. S., Ciaccio, R., … Penn, L. Z. (2021). MYC protein interactors in gene transcription and cancer. Nature Reviews Cancer, 21(9), 579–591. doi:10.1038/s41568-021-00367-9
DLBCL is the most common subtype of Non-Hodgkin Lymphoma. DLBCL starts in white blood cells called lymphocytes and it usually grows in lymph nodes. Every year, approximately ~18,000 people in the U.S. are diagnosed with DLBCL (Source: NCI SEER Data). Following initial treatment with standard chemotherapy, approximately 70% of patients have a complete response and approximately 50% of patients are cured. There is a substantial unmet need for patients with relapsed or refractory DLBCL as median overall survival is between 6-7 months in this group. Double Hit Lymphomas (DHL), Triple Hit Lymphomas (THL), and Double Expressor Lymphomas (DEL) are high grade B-cell lymphomas (HGBLs) that have high MYC and BCL2 dependency. Based on their aggressive nature, DHL, THL, and DEL represent a large portion of the relapsed or refractory DLBCL population.
MM is a cancer of plasma cells, which make antibodies (immunoglobulins) and are mainly located in the bone marrow. As cancerous cells migrate from the bone marrow, organ damage due to excess immunoglobulins in bones and blood and weakening of bones are common features. Approximately 35,000 people in the U.S. are diagnosed with MM each year and the 5-year relative survival rate is ~56% (Source: NCI SEER Data). While many therapeutic options are available to patients, a subset of highly treatment refractory patients exists. In these patients, overall survival is as low as 6 months. Additionally, it is estimated that more than 60% of MM patients have menin dependent genetic drivers (MYC addicted or driven) and that these drivers are more common in the relapsed or refractory setting.
Non-Small Cell Lung Cancer is the most common form of lung cancer, representing ~84% of all lung cancer cases or approximately 200,000 cases in the U.S. each year (Source: NCI SEER Data). Additionally, the five-year survival rate of NSCLC is ~25%. While lung cancer is the 3rd most common form of cancer in the U.S. based on incidence, lung cancer contributes to the highest number of annual cancer deaths in the U.S.. KRAS is a key node in the RAS signaling pathway, which can be oncogenic. KRAS is the most frequent oncogene in NSCLC, occurring in ~30% of patients with NSCLC. Notably, RAS signaling is known to result in active MYC, which can facilitate pro-tumor transcriptional processes. KRAS inhibitors have shown efficacy in KRAS mutant NSCLC patients in clinical trials.
Pancreatic cancer is a relatively rare form of cancer in the U.S., representing approximately 60,000 cases in the U.S. each year (Source: NCI SEER Data). Pancreatic cancer is an aggressive cancer with a very low five-year survival rate of ~11%, indicating that there is a large unmet need. It is rarely diagnosed early, contributing to the low survival rate. Among patients with pancreatic cancer, RAS mutations (including KRAS) occur in up to approximately 98% of patients.
Colorectal cancer is the fourth most common form of cancer in the U.S., representing approximately 150,000 cases in the U.S. each year (Source: NCI SEER Data). These cancers start in the rectum or the colon and can be diagnosed/identified early, even potentially as noncancerous polyps. The five-year survival rate of CRC is approximately 65%. Among other mutations, KRAS mutations occur in approximately 50% of patients with CRC.
Menin & Beta Cell Biology – Diabetes
Diabetes mellitus is characterized by a reduced ability to produce insulin and/or by a dysregulated response to insulin and affects nearly 34 million people in the U.S. (Source: CDC). Diabetes is grouped into a few clinical categories based on etiology or timing of diagnosis according to the latest guidance from the American Diabetes Association. Accounting for 1.6 million diagnosed patients in the U.S., Type 1 diabetes is due to autoimmune beta-cell destruction, usually leading to absolute insulin deficiency, including latent autoimmune diabetes of adulthood. Type 2 diabetes has been diagnosed in approximately 25.3 million people in the U.S. and is due to a progressive loss of adequate beta-cell insulin secretion frequently on the background of insulin resistance. The primary treatment goal is to achieve glycemic control by reducing HbA1c (A1c), a marker for the amount of sugar in the bloodstream, to 6.5% or lower. Glycemic control is a validated approach to delaying disease progression, which leads to significant and potentially fatal renal, cardiac, neurological, and ophthalmic comorbidities.
Loss of functional Beta-cell mass is a core component of the natural history in both types of diabetes — type 1 diabetes (mediated by autoimmune dysfunction) and type 2 diabetes (mediated by metabolic dysfunction). Beta-cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, Beta-cell mass and function are diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on Beta-cell turnover / Beta-cell growth, supporting the notion that inhibition of menin could lead to the regeneration of normal healthy Beta-cells. Notably, it has previously been shown that knocking out the gene responsible for the creation of menin (MEN1) has been observed to produce profound glycemic control in diabetic animal models (see Figure below). Based on these and other scientific findings, Biomea explored the potential for menin inhibition as a viable therapeutic approach to permanently halt or reverse progression of type 2 diabetes.
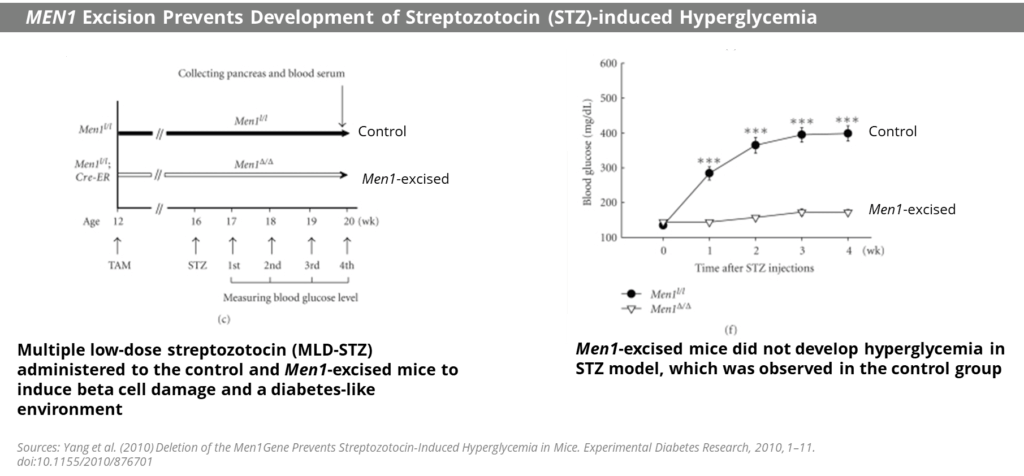
Fig. MEN1 knockdown led to profound glycemic control in a streptozotocin-induced hyperglycemia mouse model
BMF-219’s proposed mechanism of action in diabetes is to enable the proliferation, preservation, and reactivation of a patient’s own healthy, functional, insulin-producing beta cells. As the potentially first disease-modifying therapy for type 2 diabetes, BMF-219 could be an important addition and complement to the treatment landscape, if approved.
In March 2023, Biomea reported initial clinical data from the first two cohorts of the Phase II portion of COVALENT-111 (NCT05731544). As reported, 89% of patients enrolled in Cohort 3 (n=10 patients at 100 mg without food) achieved a reduction in HbA1c, 78% achieved ≥ 0.5% reduction in HbA1c and 56% achieved ≥ 1% reduction in HbA1c (median and mean reduction over the cohort: -1.0% and -0.81%, respectively). BMF-219 was well tolerated and demonstrated a favorable safety profile with no dose discontinuations.
In June 2023, additional clinical data was presented from the first two cohorts of patients with T2D enrolled in the Phase II portion of COVALENT-111. New data highlighted specific patients treated with BMF-219 for 4 weeks maintained or experienced a further decrease in HbA1c levels 8 weeks after treatment was completed, up to a 2.4% reduction from baseline. ( Rodriguez et al. ADA 2023 )
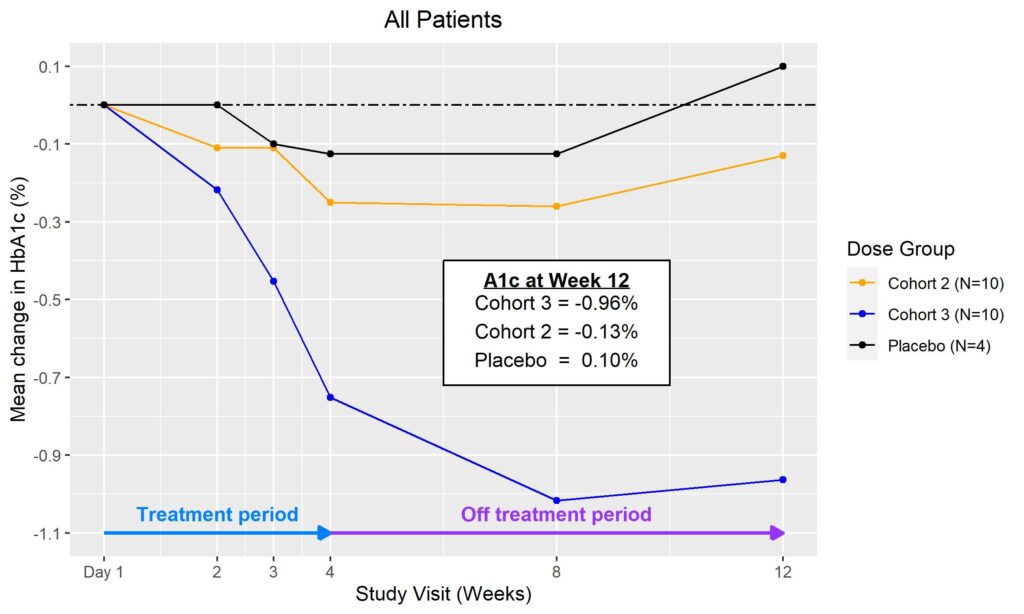
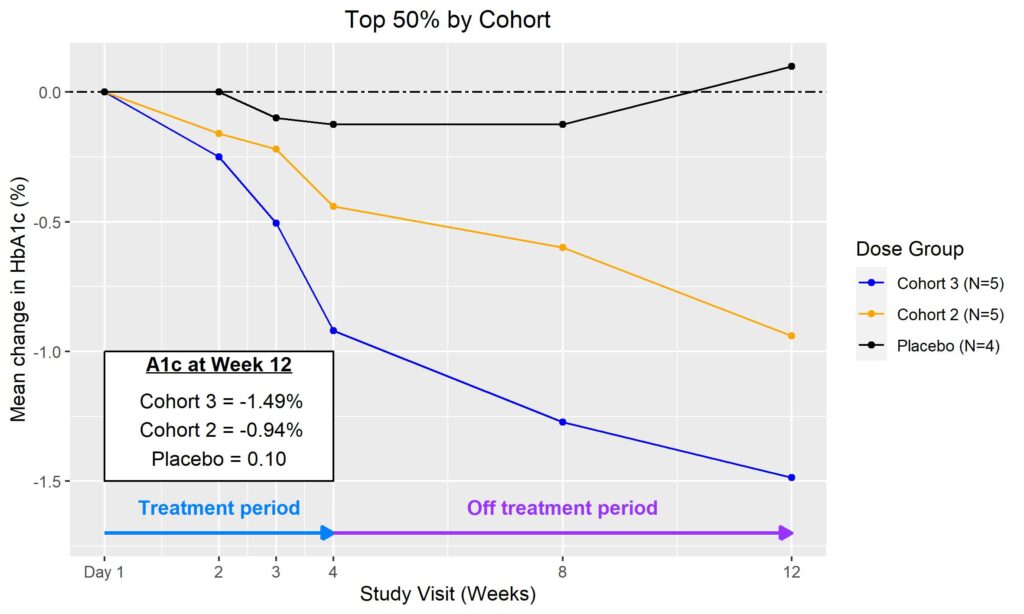
Fig. Clinical data presented at 2023 ADA. The top 50% of responders after 4-weeks of treatment in Cohorts 2 and 3 (100 mg BMF-219 fed and fasted, respectively) demonstrated durable and ongoing reduction in HbA1c while off treatment up to Week 12; a continued reduction in HbA1c was observed in Cohort 2 (additional 114%) and in Cohort 3 (additional 62%) (Rodriguez et al. ADA 2023)
Biomea Films:
ADA 2023: BMF219-Addressing the Root Cause of Diabetes in Clinical Trials
The American Diabetes Association (ADA) featured Biomea during its yearly “Thought Leadership Film Series” of innovative therapies at its 83rd Annual Meeting.
ADA 2022: BMF219 Preclinical Development Progress
The American Diabetes Association (ADA) featured Biomea during its yearly “Thought Leadership Film Series” of innovative therapies at its 82nd Annual Meeting.
BMF-500 in AML
BMF-500 is an investigational, novel, orally bioavailable, highly potent and selective covalent small molecule inhibitor of fms-like tyrosine kinase 3 (FLT3). Biomea has continued advancing BMF-500 toward the clinic, with investigational new drug (IND) application cleared in 1H2023 and initiated a Phase I study of BMF-500 (COVALENT-103; NCT05918692).
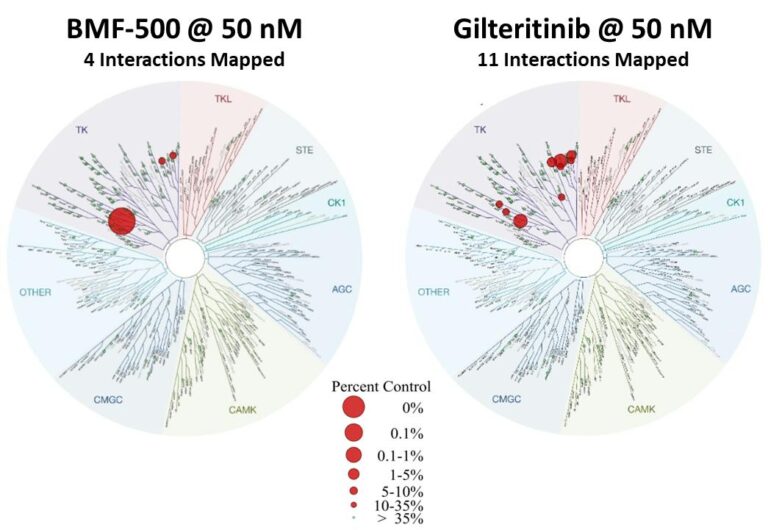
Fig. BMF-500 is highly selective to FLT3 (Law et al. ASH 2022).
BMF-500 was discovered and developed in-house at Biomea using the company’s proprietary FUSION™ System and has demonstrated best-of-class potential based on extensive preclinical studies. The kinase profile of BMF-500 showed high target selectivity, suggesting the potential for minimal off-target liabilities. BMF-500 was designed to have a therapeutic profile to allow for combinations with standard of care and/or novel targeted agents like BMF-219.
FLT3 is a receptor tyrosine kinase (RTK) that plays a central role in the survival, proliferation, and differentiation of immature blood cells. Notably, FLT3 gene mutations are common in patients with AML and are associated with a poor prognosis. Nearly 30% of AML patients have a FLT3 mutation, representing more than 6,000 incident patients in the United States each year. While FLT3-specific and pan-tyrosine kinase inhibitors are approved by the FDA across various lines of therapy in AML, these agents have produced relatively low rates of durable responses and overall survival remains an unmet need.
Previous data presented at medical conferences showed BMF-500’s picomolar affinity to activating FLT3 mutations, including FLT3-ITD and various tyrosine kinase domain (TKD) mutations (Law et al. ASH 2022). BMF-500 demonstrated multi-fold higher potency and increased cytotoxicity than commercially available non-covalent FLT3 inhibitor gilteritinib. Further data also exhibited the potential utility of combination strategies to achieve higher antileukemic cell killing with reduced concentrations of BMF-500 and BMF-219 (Law et al. AACR 2023). These data provide preclinical evidence for combining pathway-specific inhibitors as a promising therapeutic strategy for further investigation in acute leukemia.
