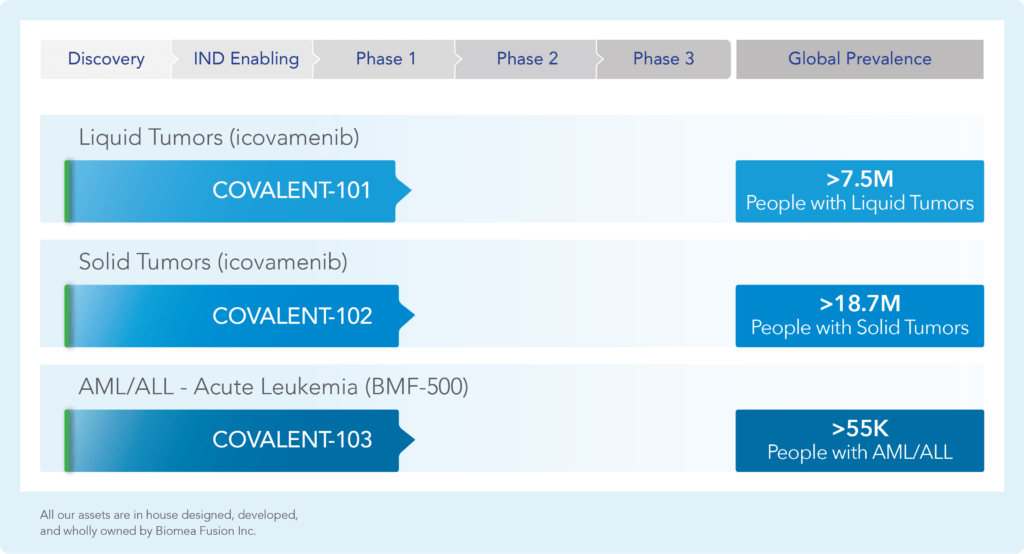
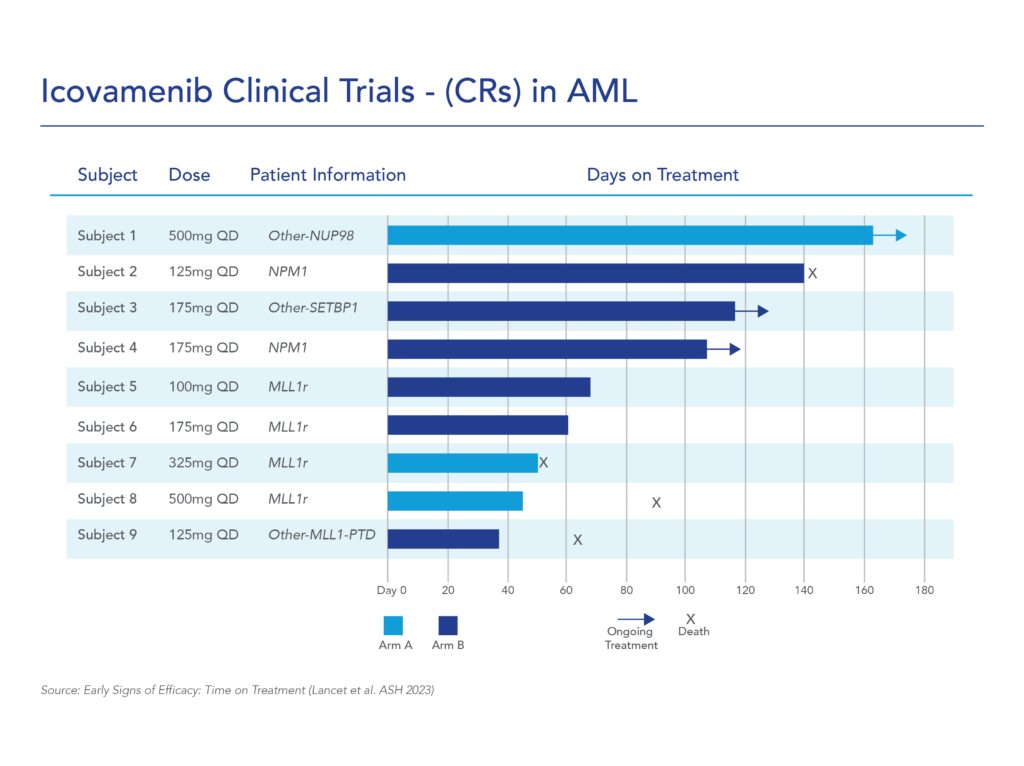
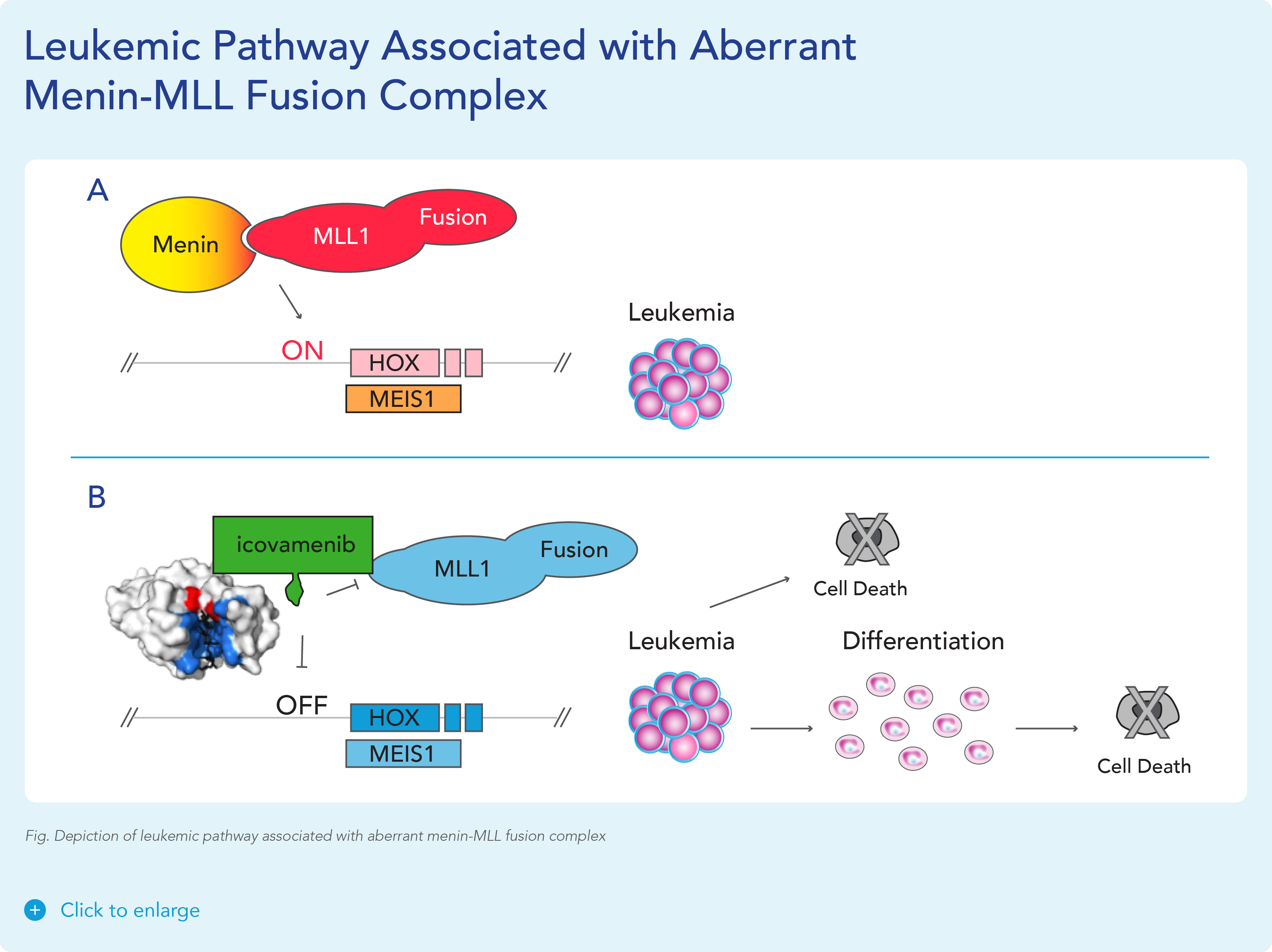
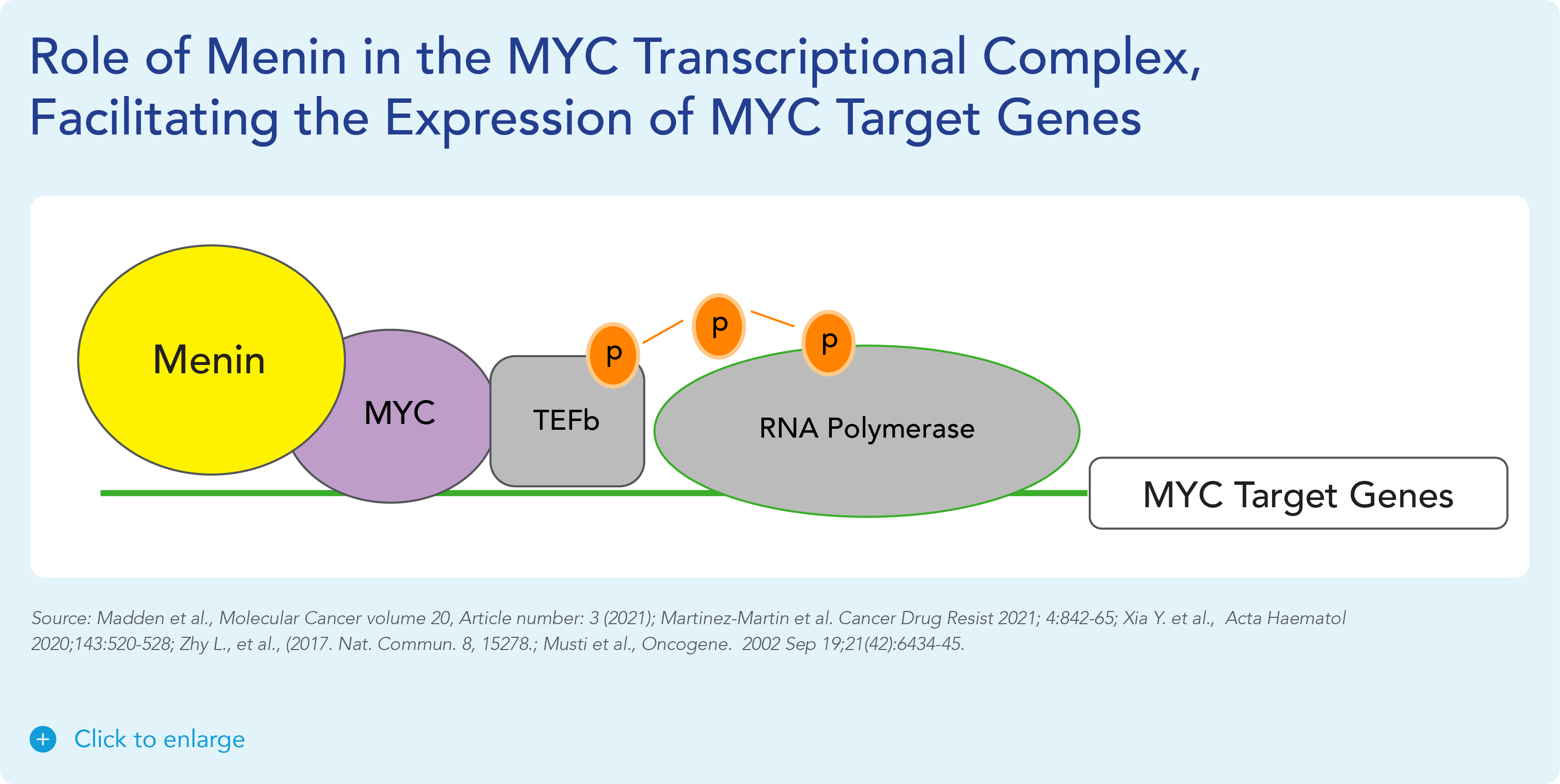
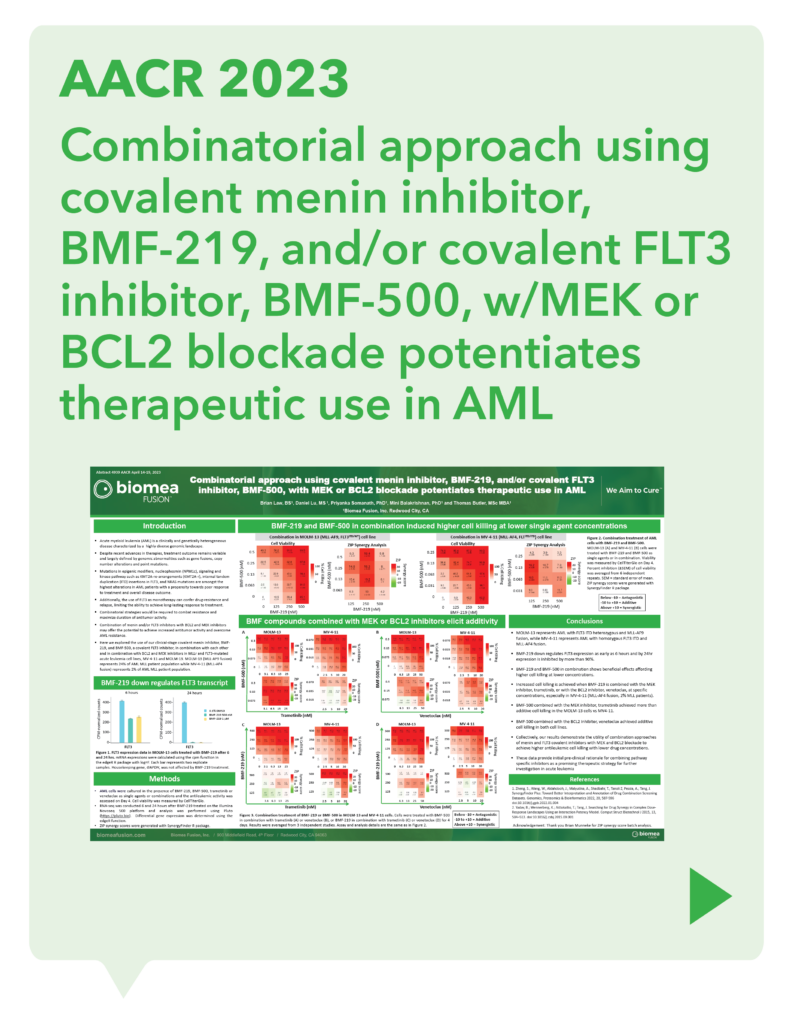
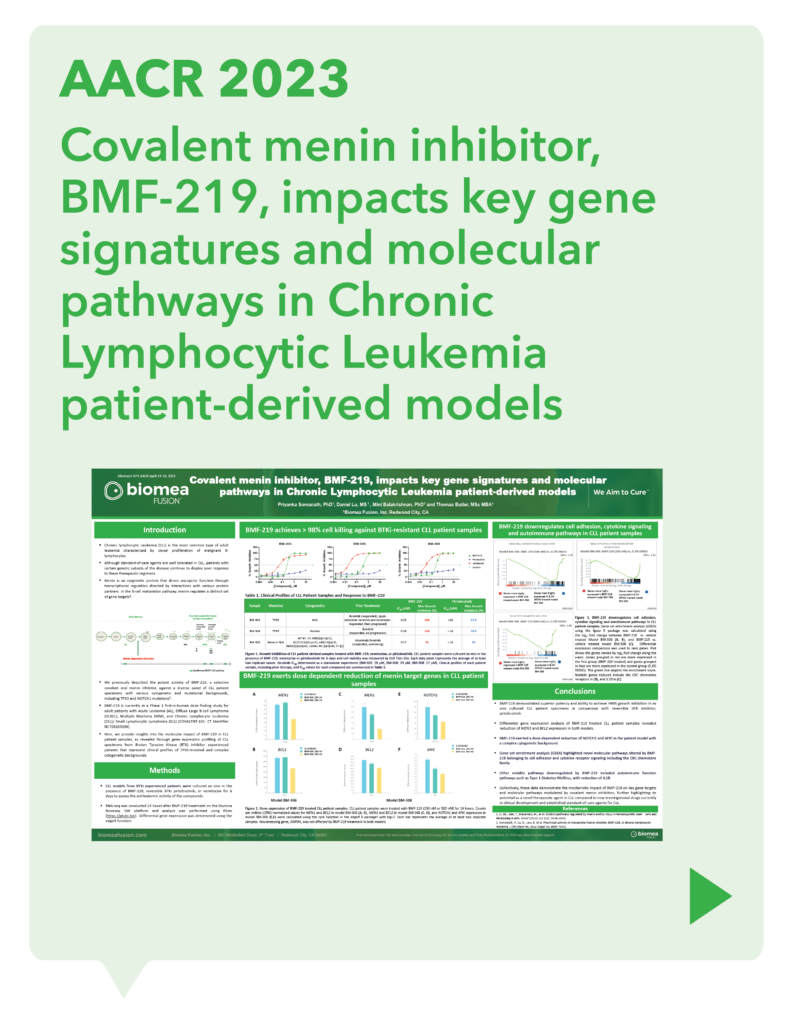
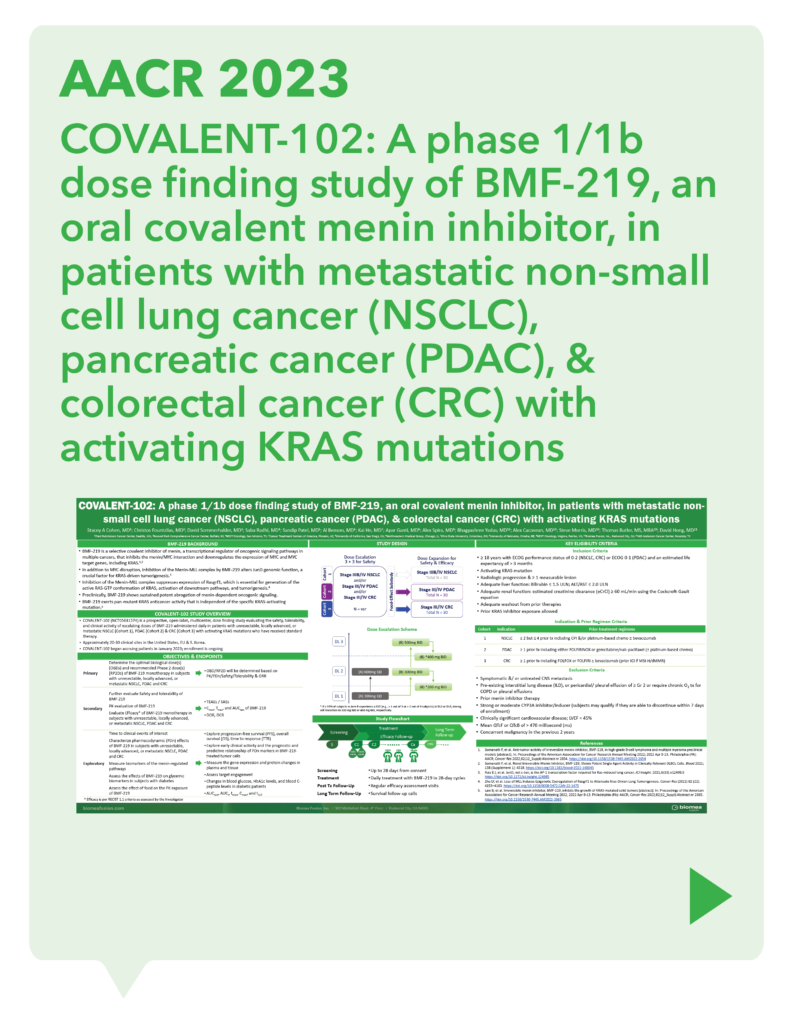
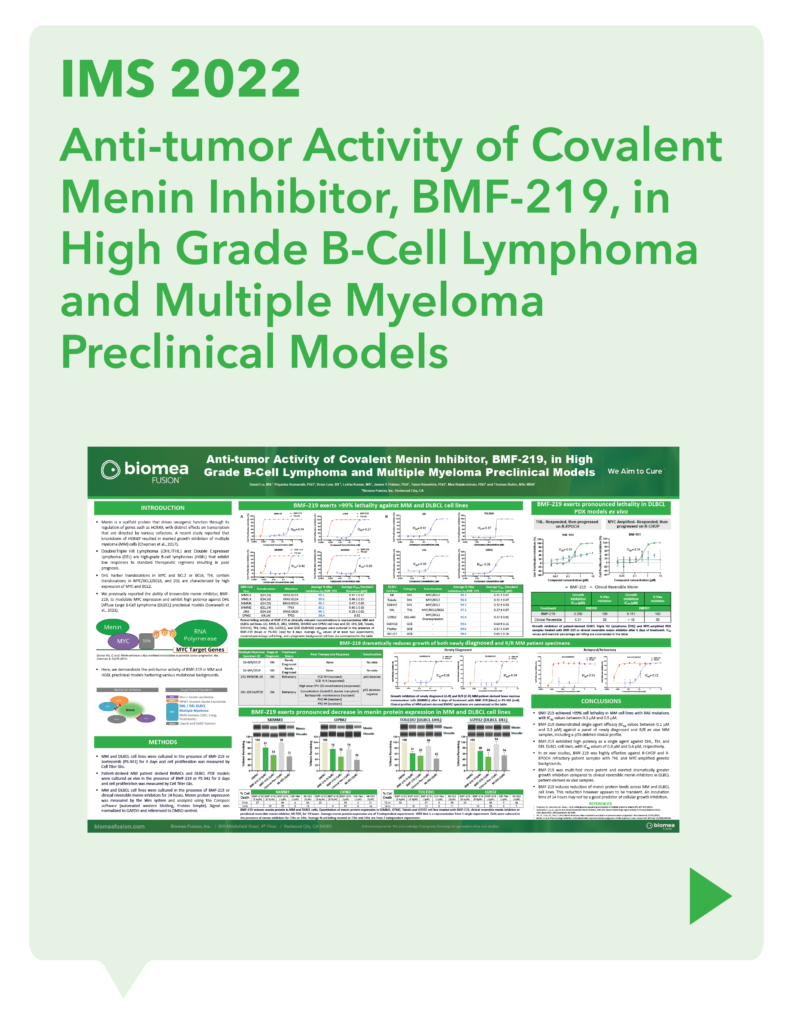
var prData = {"links":{"self":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news","first":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=1","next":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=2","prev":null,"last":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=2"},"meta":{"executionDate":"2025-01-22T04:42:44","cmsDomain":"https://investors.biomeafusion.com","count":100},"data":[{"id":9256,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9256","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-become-diabetes-obesity-medicines-company"},"title":"Biomea Fusion to Become a Diabetes & Obesity Medicines Company","type":{"title":"General","id":3886},"teaser":"Icovamenib & BMF-650 (oral small molecule GLP-1) are the cornerstones of the metabolic franchise Biomea preparing icovamenib for late-stage clinical development 2025 corporate update to be presented at the 43 rd Annual J.P. Morgan Healthcare Conference REDWOOD CITY, Calif., Jan.","language":"en","releaseDate":{"dateUTC":"2025-01-13T14:00:37","date":"2025-01-13T09:00:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Become a Diabetes & Obesity Medicines Company","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9256/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-13T14:00:41","lastUpdatedUTC":"2025-01-13T14:00:41"},{"id":9246,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9246","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-new-preclinical-data-icovamenib"},"title":"Biomea Fusion Reports New Preclinical Data on Icovamenib-Semaglutide Combination Study","type":{"title":"General","id":3886},"teaser":"New in vivo preclinical data announced today, demonstrate that icovamenib, in combination with semaglutide showed additional 11.5% body weight reduction and 43% increase in lean muscle mass compared to semaglutide alone Icovamenib, in combination with semaglutide, approximately doubled C-peptide","language":"en","releaseDate":{"dateUTC":"2025-01-07T14:00:58","date":"2025-01-07T09:00:58","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports New Preclinical Data on Icovamenib-Semaglutide Combination Study","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9246/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-07T14:01:00","lastUpdatedUTC":"2025-01-07T14:01:00"},{"id":9231,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9231","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-43rd-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at the 43rd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 06, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2025-01-06T12:00:37","date":"2025-01-06T07:00:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 43rd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9231/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-06T12:00:43","lastUpdatedUTC":"2025-01-06T12:00:43"},{"id":9211,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9211","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-positive-topline-results-ongoing-phase"},"title":"Biomea Fusion Announces Positive Topline Results from Ongoing Phase II COVALENT-111 Study in Patients with Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Icovamenib met the primary endpoint, displaying a meaningful statistically significant placebo-corrected mean reduction in HbA1c in the prespecified per protocol patient population Best response achieved in target, beta-cell deficient patients on one or more antidiabetic agents at baseline, showing","language":"en","releaseDate":{"dateUTC":"2024-12-17T13:10:38","date":"2024-12-17T08:10:38","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Positive Topline Results from Ongoing Phase II COVALENT-111 Study in Patients with Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9211/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-17T13:10:43","lastUpdatedUTC":"2024-12-17T13:10:43"},{"id":9201,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9201","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-announce-topline-results"},"title":"Biomea Fusion to Host Conference Call to Announce Topline Results from Phase II COVALENT-111 Study in Patients with Type 2 Diabetes (T2D)","type":{"title":"General","id":3886},"teaser":"Tuesday, December 17, 2024 at 8:00 am EST REDWOOD CITY, Calif., Dec. 16, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of","language":"en","releaseDate":{"dateUTC":"2024-12-16T23:16:01","date":"2024-12-16T18:16:01","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call to Announce Topline Results from Phase II COVALENT-111 Study in Patients with Type 2 Diabetes (T2D)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9201/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-16T23:16:04","lastUpdatedUTC":"2024-12-16T23:16:04"},{"id":9166,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9166","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-oral-and-poster-presentations-icovamenib"},"title":"Biomea Fusion Announces Oral and Poster Presentations of Icovamenib at the 22nd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"In preclinical experiments, icovamenib enhanced beta cell function and responsiveness of human islets to GLP-1-based therapies. These effects were associated with an increase in the expression levels of both the GLP-1 receptor (GLP-1R) as well as intracellular insulin.","language":"en","releaseDate":{"dateUTC":"2024-12-12T22:02:59","date":"2024-12-12T17:02:59","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Oral and Poster Presentations of Icovamenib at the 22nd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9166/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-12T22:03:04","lastUpdatedUTC":"2024-12-12T22:03:04"},{"id":9156,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9156","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-preliminary-data-ongoing-covalent-103"},"title":"Biomea Fusion Announces Preliminary Data from Ongoing COVALENT-103 Study of Investigational Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"Preliminary data supports BMF-500’s potential as a transformative therapy for patients with FLT3 mutated relapsed or refractory (R/R) acute leukemia BMF-500 showed a favorable safety and tolerability profile, with no dose-limiting toxicities observed across all dose levels Pharmacokinetic and","language":"en","releaseDate":{"dateUTC":"2024-12-09T21:01:30","date":"2024-12-09T16:01:30","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Preliminary Data from Ongoing COVALENT-103 Study of Investigational Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9156/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-09T21:01:39","lastUpdatedUTC":"2024-12-09T21:01:39"},{"id":9136,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9136","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-present-initial-clinical-data"},"title":"Biomea Fusion to Host Conference Call to Present initial Clinical Data from Phase I COVALENT-103 Study of BMF-500, a Covalent FLT3 Inhibitor, in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Dec. 06, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2024-12-06T13:00:32","date":"2024-12-06T08:00:32","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call to Present initial Clinical Data from Phase I COVALENT-103 Study of BMF-500, a Covalent FLT3 Inhibitor, in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9136/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-06T13:00:34","lastUpdatedUTC":"2024-12-06T13:00:34"},{"id":9121,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9121","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-2"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Dec. 02, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of oral covalent small molecules to treat and improve the lives of patients with diabetes,","language":"en","releaseDate":{"dateUTC":"2024-12-02T21:05:31","date":"2024-12-02T16:05:31","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9121/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-02T21:05:36","lastUpdatedUTC":"2024-12-02T21:05:36"},{"id":9056,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9056","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/late-breaker-oral-presentation-showing-new-analysis-escalation"},"title":"Late-Breaker Oral Presentation Showing New Analysis from the Escalation Portion of COVALENT-111 Presented at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","type":{"title":"General","id":3886},"teaser":"Icovamenib Achieves a Mean Reduction in HbA1c Greater than 1% at Week 26 Following 4 Weeks of Dosing in Type 2 Diabetes Patients Characterized by Insulin Deficiency 32 patients from the 100mg and 200mg cohorts, doses selected for the expansion phase, were characterized based on baseline biomarkers","language":"en","releaseDate":{"dateUTC":"2024-11-18T13:00:29","date":"2024-11-18T08:00:29","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Late-Breaker Oral Presentation Showing New Analysis from the Escalation Portion of COVALENT-111 Presented at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9056/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-11-18T13:00:36","lastUpdatedUTC":"2024-11-18T13:00:36"},{"id":9041,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9041","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-1"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Nov. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of oral covalent small molecules to treat and improve the lives of patients with diabetes,","language":"en","releaseDate":{"dateUTC":"2024-11-01T20:06:18","date":"2024-11-01T16:06:18","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9041/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-11-01T20:06:25","lastUpdatedUTC":"2024-11-01T20:06:25"},{"id":9016,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9016","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-preclinical-data-showing-icovamenib-bmf"},"title":"Biomea Fusion Presents Preclinical Data Showing Icovamenib (BMF-219) Enhanced Effectiveness of GLP-1-Based Therapies and Introduces BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist Candidate","type":{"title":"General","id":3886},"teaser":"Preclinical data from ex vivo human islet experiments showed that icovamenib (BMF-219) was able to enhance the activity of glucagon-like peptide-1 (GLP-1)-based therapies, potentially leading to increased insulin secretion and improved glycemic control in patients with diabetes Phase II study","language":"en","releaseDate":{"dateUTC":"2024-10-30T20:01:10","date":"2024-10-30T16:01:10","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Preclinical Data Showing Icovamenib (BMF-219) Enhanced Effectiveness of GLP-1-Based Therapies and Introduces BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist Candidate","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9016/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-30T20:01:21","lastUpdatedUTC":"2024-10-30T20:01:21"},{"id":8996,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8996","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"U.S. Food and Drug Administration (FDA) lifted Clinical Hold on COVALENT-111 (Type 2 Diabetes) & COVALENT-112 (Type 1 Diabetes) trials COVALENT-111 Phase 2b 26-week topline data of the dose expansion cohorts expected in December 2024 COVALENT-112 Phase 2a 26-week topline data of the open label","language":"en","releaseDate":{"dateUTC":"2024-10-29T20:46:46","date":"2024-10-29T16:46:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8996/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-29T20:46:51","lastUpdatedUTC":"2024-10-29T20:46:51"},{"id":8981,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8981","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-approval-icovamenib-international"},"title":"Biomea Fusion Announces Approval of “icovamenib” as International Nonproprietary Name for BMF-219","type":{"title":"General","id":3886},"teaser":"Icovamenib is an oral covalent menin inhibitor in clinical development to investigate its impact on the function of insulin-producing beta cells REDWOOD CITY, Calif., Oct. 21, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company","language":"en","releaseDate":{"dateUTC":"2024-10-21T13:00:57","date":"2024-10-21T09:00:57","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Approval of “icovamenib” as International Nonproprietary Name for BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8981/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-21T13:01:01","lastUpdatedUTC":"2024-10-21T13:01:01"},{"id":8971,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8971","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-and-webcast-wednesday-october"},"title":"Biomea Fusion to Host Conference Call and Webcast on Wednesday, October 30th at 4:30 pm ET to Announce Our Lead Clinical Candidate, BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist and Preclinical Data Combining BMF-219 with a GLP-1 RA-Based Therapy","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Oct. 15, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2024-10-15T20:05:48","date":"2024-10-15T16:05:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call and Webcast on Wednesday, October 30th at 4:30 pm ET to Announce Our Lead Clinical Candidate, BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist and Preclinical Data Combining BMF-219 with a GLP-1 RA-Based Therapy","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8971/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-15T20:05:57","lastUpdatedUTC":"2024-10-15T20:05:57"},{"id":8956,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8956","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-1st-annual-asian-conference-innovative"},"title":"Biomea Fusion to Present at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational oral covalent menin inhibitor developed to regenerate insulin-producing beta cells Two trial-in-progress oral presentations to feature Phase II study design of oral covalent menin inhibitor BMF-219 in patients with type 2 diabetes (COVALENT-111) and in patients with","language":"en","releaseDate":{"dateUTC":"2024-10-07T13:01:09","date":"2024-10-07T09:01:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8956/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-07T13:01:11","lastUpdatedUTC":"2024-10-07T13:01:11"},{"id":8931,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8931","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-announces-formation-global-scientific-advisory-board-22"},"title":"Biomea Announces Formation of Global Scientific Advisory Board with 22 World-Renowned Diabetes Experts","type":{"title":"General","id":3886},"teaser":"International diabetes pioneers and thought leaders from 11 countries will work with Biomea leadership to unlock the potential of menin science and beta cell biology to advance BMF-219 and Biomea’s evolving pipeline REDWOOD CITY, Calif., Oct. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2024-10-01T13:00:29","date":"2024-10-01T09:00:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Announces Formation of Global Scientific Advisory Board with 22 World-Renowned Diabetes Experts","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8931/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-01T13:00:39","lastUpdatedUTC":"2024-10-01T16:58:29"},{"id":8921,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8921","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/fda-lifts-clinical-hold-bmf-219-type-2-and-type-1-diabetes"},"title":"FDA Lifts Clinical Hold on BMF-219 in Type 2 and Type 1 Diabetes Trials","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Sept. 26, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with metabolic","language":"en","releaseDate":{"dateUTC":"2024-09-26T18:01:15","date":"2024-09-26T14:01:15","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"FDA Lifts Clinical Hold on BMF-219 in Type 2 and Type 1 Diabetes Trials","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8921/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-09-26T18:01:25","lastUpdatedUTC":"2024-09-26T18:01:25"},{"id":8806,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8806","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-0"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Aug. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with metabolic diseases and genetically defined","language":"en","releaseDate":{"dateUTC":"2024-08-01T20:05:36","date":"2024-08-01T16:05:36","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8806/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-08-01T20:05:44","lastUpdatedUTC":"2024-08-01T20:05:44"},{"id":8791,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8791","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"COVALENT-111 Phase 2b on track for Q4 2024 readout COVALENT-112 Phase 2a on track for Q4 2024 readout Announcement of the third program in obesity on track for Q3 2024 REDWOOD CITY, Calif., July 31, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or “the Company”) (Nasdaq: BMEA), a","language":"en","releaseDate":{"dateUTC":"2024-07-31T20:06:04","date":"2024-07-31T16:06:04","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8791/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-07-31T20:06:13","lastUpdatedUTC":"2024-07-31T20:06:13"},{"id":8721,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8721","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-bmf-219-diabetes-placed-clinical-hold"},"title":"Biomea Fusion Announces BMF-219 in Diabetes Placed on Clinical Hold ","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 06, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), announced that the Company has received notice from the U.S. Food and Drug Administration (FDA) that a full clinical hold has been placed on Biomea’s ongoing Phase I/II clinical","language":"en","releaseDate":{"dateUTC":"2024-06-06T20:06:41","date":"2024-06-06T16:06:41","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces BMF-219 in Diabetes Placed on Clinical Hold ","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8721/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-06-06T20:06:51","lastUpdatedUTC":"2024-06-06T20:06:51"},{"id":8711,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8711","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-listing"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 03, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-06-03T20:05:58","date":"2024-06-03T16:05:58","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8711/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-06-03T20:06:04","lastUpdatedUTC":"2024-06-03T20:06:04"},{"id":8701,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8701","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-completion-enrollment-first-3-arms-phase"},"title":"Biomea Fusion Announces Completion of Enrollment of First 3 Arms in Phase 2 Expansion Cohorts of COVALENT-111 Study for BMF-219 in Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells REDWOOD CITY, Calif., May 30, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing","language":"en","releaseDate":{"dateUTC":"2024-05-30T13:12:58","date":"2024-05-30T09:12:58","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Completion of Enrollment of First 3 Arms in Phase 2 Expansion Cohorts of COVALENT-111 Study for BMF-219 in Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8701/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-05-30T13:13:07","lastUpdatedUTC":"2024-05-30T13:13:07"},{"id":8671,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8671","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported positive data from the escalation portion of Phase 1/2 study (COVALENT-111) in type 2 diabetes patients, displaying durable improved glycemic control while off therapy for 22 weeks, supporting the disease-modifying potential of BMF-219 to address a root cause of diabetes: a loss of","language":"en","releaseDate":{"dateUTC":"2024-05-02T20:05:27","date":"2024-05-02T16:05:27","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8671/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-05-02T20:05:36","lastUpdatedUTC":"2024-05-02T20:05:36"},{"id":8631,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8631","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grants-under-nasdaq-0"},"title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., April 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-04-01T20:11:09","date":"2024-04-01T16:11:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8631/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T20:11:11","lastUpdatedUTC":"2024-04-01T20:11:11"},{"id":8616,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8616","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-highlights-initial-data-first-two-type-1-diabetes"},"title":"Biomea Fusion Highlights Initial Data from the First Two Type 1 Diabetes Patients Dosed with BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes The first two type 1 diabetes patients enrolled in COVALENT-112 both demonstrated early signs of clinical activity with improved measures of beta-cell","language":"en","releaseDate":{"dateUTC":"2024-04-01T13:02:44","date":"2024-04-01T09:02:44","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Highlights Initial Data from the First Two Type 1 Diabetes Patients Dosed with BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8616/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T13:02:53","lastUpdatedUTC":"2024-04-01T13:02:53"},{"id":8601,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8601","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2023"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"In 2023, reported Phase 2 data (COVALENT-111) in type 2 diabetes patients supporting the disease-modifying potential of BMF-219 to address a root cause of diabetes: a loss of healthy, insulin-producing beta cells. After just a 4-week treatment period in type 2 diabetes patients, who had previously","language":"en","releaseDate":{"dateUTC":"2024-04-01T13:00:37","date":"2024-04-01T09:00:37","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8601/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T13:00:43","lastUpdatedUTC":"2024-04-01T13:00:43"},{"id":8581,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8581","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-patient-cohorts-covalent-111-displaying"},"title":"Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function","type":{"title":"General","id":3886},"teaser":"Three Clinical Data Sets from the Dose Escalation Phase of COVALENT-111 to be Presented at the 17 th Annual ATTD Conference Highlighting BMF-219’s Novel Mechanism of Action in Patients with Type 2 Diabetes Patients in COVALENT-111 are displaying improved glycemic control while off therapy out to","language":"en","releaseDate":{"dateUTC":"2024-03-06T08:05:37","date":"2024-03-06T03:05:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8581/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-03-06T08:05:45","lastUpdatedUTC":"2024-03-06T08:05:45"},{"id":8551,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8551","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grants-under-nasdaq-listing"},"title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-03-01T21:16:53","date":"2024-03-01T16:16:53","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8551/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-03-01T21:16:56","lastUpdatedUTC":"2024-03-01T21:16:56"},{"id":8451,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8451","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-highlights-recent-updates-and-anticipated-2024"},"title":"Biomea Fusion Highlights Recent Updates and Anticipated 2024 Corporate Milestones at 42nd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"Expansion portion of Phase II COVALENT-111 study readout in 216 patients with type 2 diabetes expected in 2024. Open label portion of Phase II COVALENT-112 study readout in 40 patients with type 1 diabetes expected in 2024. REDWOOD CITY, Calif., Jan. 09, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2024-01-09T16:03:07","date":"2024-01-09T11:03:07","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Highlights Recent Updates and Anticipated 2024 Corporate Milestones at 42nd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8451/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-09T16:03:08","lastUpdatedUTC":"2024-01-09T16:03:08"},{"id":8446,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8446","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-type-1-diabetes-patient"},"title":"Biomea Fusion Announces Dosing of First Type 1 Diabetes Patient in Phase II Study (COVALENT-112) with BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes The clinical study, COVALENT-112, has initiated enrollment of adults living with type 1 diabetes in the US and Canada.","language":"en","releaseDate":{"dateUTC":"2024-01-08T14:01:49","date":"2024-01-08T09:01:49","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Type 1 Diabetes Patient in Phase II Study (COVALENT-112) with BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8446/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-08T14:01:55","lastUpdatedUTC":"2024-01-08T14:01:55"},{"id":8421,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8421","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-42nd-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at the 42nd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 02, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2024-01-02T21:05:37","date":"2024-01-02T16:05:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 42nd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8421/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-02T21:05:45","lastUpdatedUTC":"2024-01-02T21:05:45"},{"id":8396,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8396","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-achievement-minimal-residual-disease"},"title":"Biomea Fusion Presents Achievement of Minimal Residual Disease Negativity (MRD-neg) in First Complete Responder from Ongoing Phase I Study (COVALENT-101) of BMF-219 in Patients with Relapsed or Refractory (R/R) Acute Myeloid Leukemia (AML) at the 2023 ASH","type":{"title":"General","id":3886},"teaser":"BMF-219 demonstrated early signs of clinical activity and ability to achieve durable and sustained complete responses (CRs) with minimal residual disease negativity (MRD-neg) in acute myeloid leukemia (AML) patients Pharmacodynamic data further supports the mechanism of action of BMF-219 as a menin","language":"en","releaseDate":{"dateUTC":"2023-12-11T14:25:47","date":"2023-12-11T09:25:47","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Achievement of Minimal Residual Disease Negativity (MRD-neg) in First Complete Responder from Ongoing Phase I Study (COVALENT-101) of BMF-219 in Patients with Relapsed or Refractory (R/R) Acute Myeloid Leukemia (AML) at the 2023 ASH","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8396/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-11T14:25:55","lastUpdatedUTC":"2023-12-11T14:25:55"},{"id":8376,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8376","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-near-doubling-percentage-patients"},"title":"Biomea Fusion Announces Near Doubling the Percentage of Patients with Durable HbA1c Reduction in the 200 mg Dose Cohorts","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes At Week 26, 22 weeks after the last dose of BMF-219, the 200 mg cohorts increased the percentage of patients to approximately 40% with durable HbA1c","language":"en","releaseDate":{"dateUTC":"2023-12-09T22:30:45","date":"2023-12-09T17:30:45","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Near Doubling the Percentage of Patients with Durable HbA1c Reduction in the 200 mg Dose Cohorts","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8376/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-09T22:30:55","lastUpdatedUTC":"2023-12-09T22:30:55"},{"id":8356,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8356","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-long-term-follow-data-showing-improved"},"title":"Biomea Fusion Presents Long-Term Follow-Up Data Showing Improved Glycemic Control after 22 Weeks Off Treatment in Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes in a Poster Presentation at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes At Week 26, 22 weeks after the last dose of BMF-219, participants in the 100 mg QD (without food) cohort saw an improved placebo adjusted mean reduction in","language":"en","releaseDate":{"dateUTC":"2023-12-08T14:00:12","date":"2023-12-08T09:00:12","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Long-Term Follow-Up Data Showing Improved Glycemic Control after 22 Weeks Off Treatment in Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes in a Poster Presentation at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8356/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-08T14:00:16","lastUpdatedUTC":"2023-12-08T14:12:32"},{"id":8346,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8346","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-long-term-follow-data-ongoing-phase-ii"},"title":"Biomea Fusion to Present Long-Term Follow-Up Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes and Results from Ex-Vivo Human Islet Experiments at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes In a poster presentation on Thursday, Dec. 7 th at 6:30pm and an oral presentation on Friday, Dec. 8 th at 7:30pm, Biomea to present new preclinical and","language":"en","releaseDate":{"dateUTC":"2023-12-07T14:15:42","date":"2023-12-07T09:15:42","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Long-Term Follow-Up Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes and Results from Ex-Vivo Human Islet Experiments at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8346/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-07T14:15:45","lastUpdatedUTC":"2023-12-07T17:50:25"},{"id":8331,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8331","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-health-canada-clearance-clinical-trial"},"title":"Biomea Fusion Announces Health Canada Clearance of Clinical Trial Application (CTA) for BMF-219 in Type 1 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is a novel investigational covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes Health Canada has cleared the initiation of COVALENT-112, a Phase II clinical trial of BMF-219 in adults living with type 1 diabetes (T1D), following FDA","language":"en","releaseDate":{"dateUTC":"2023-12-05T13:31:10","date":"2023-12-05T08:31:10","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Health Canada Clearance of Clinical Trial Application (CTA) for BMF-219 in Type 1 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8331/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-05T13:31:16","lastUpdatedUTC":"2023-12-05T13:31:16"},{"id":8286,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8286","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-acceptance-three-abstracts-its-novel"},"title":"Biomea Fusion Announces Acceptance of Three Abstracts for its Novel Menin Inhibitor Highlighting Durable Glycemic Control in Diabetes Patients at the International Conference on Advanced Technologies & Treatments for Diabetes (ATTD) in 2024","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes New clinical data from all dosing cohorts (n=62) initiated to date from the escalation portion of COVALENT-111 and long-term (26 weeks) follow-up data of","language":"en","releaseDate":{"dateUTC":"2023-11-27T13:31:19","date":"2023-11-27T08:31:19","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Acceptance of Three Abstracts for its Novel Menin Inhibitor Highlighting Durable Glycemic Control in Diabetes Patients at the International Conference on Advanced Technologies & Treatments for Diabetes (ATTD) in 2024","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8286/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-27T13:31:27","lastUpdatedUTC":"2023-11-27T13:31:27"},{"id":8276,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8276","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-abstract-bmf-219-novel-therapeutic-agent"},"title":"Biomea Fusion Abstract “BMF-219: A Novel Therapeutic Agent to Reestablish Functional Beta Cells and Provide Long-term Glycemic Control\" Selected as One of Six Oral Presentations at 21st World Congress Insulin Resistance, Diabetes & Cardiovascular Disease","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure diabetes New clinical data providing long-term 26 weeks follow-up of the initial two dosing cohorts of COVALENT-111 will be presented during the Abstract Oral","language":"en","releaseDate":{"dateUTC":"2023-11-16T13:31:35","date":"2023-11-16T08:31:35","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Abstract “BMF-219: A Novel Therapeutic Agent to Reestablish Functional Beta Cells and Provide Long-term Glycemic Control\" Selected as One of Six Oral Presentations at 21st World Congress Insulin Resistance, Diabetes & Cardiovascular Disease","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8276/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-16T13:31:39","lastUpdatedUTC":"2023-11-16T13:31:39"},{"id":8266,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8266","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-data-ongoing-phase-ii-study-covalent"},"title":"Biomea Fusion to Present New Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure type 2 diabetes An abstract, “BMF-219: A novel therapeutic agent to reestablish functional beta cells and provide long-term glycemic control” will be presented","language":"en","releaseDate":{"dateUTC":"2023-11-08T21:08:36","date":"2023-11-08T16:08:36","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8266/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-08T21:08:50","lastUpdatedUTC":"2023-11-08T21:08:50"},{"id":8256,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8256","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-two-poster-presentations-upcoming-ash"},"title":"Biomea Fusion Announces Two Poster Presentations at Upcoming ASH Annual Meeting 2023","type":{"title":"General","id":3886},"teaser":"First presentation of clinical data from ongoing COVALENT-101 trial of covalent menin inhibitor BMF-219 as a treatment for patients with liquid tumors Trial in progress presentation featuring study design of ongoing COVALENT-103 trial of covalent FLT3 inhibitor BMF-500 REDWOOD CITY, Calif., Nov.","language":"en","releaseDate":{"dateUTC":"2023-11-02T13:06:51","date":"2023-11-02T09:06:51","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Two Poster Presentations at Upcoming ASH Annual Meeting 2023","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8256/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-02T13:06:57","lastUpdatedUTC":"2023-11-02T13:06:57"},{"id":8231,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8231","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Demonstrated durable HbA1c lowering in the escalation portion of ongoing Phase II study in type 2 diabetes (COVALENT-111), with 84% of all patients showing a reduction of HbA1c after 4 weeks dosing and 74% after another 8 weeks off-treatment period Expansion portion of COVALENT-111 cleared and","language":"en","releaseDate":{"dateUTC":"2023-10-30T20:01:48","date":"2023-10-30T16:01:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8231/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-30T20:01:57","lastUpdatedUTC":"2023-10-30T20:01:57"},{"id":8226,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8226","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-covalent-flt3"},"title":"Biomea Fusion Announces First Patient Dosed with Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia in Phase I Clinical Trial (COVALENT-103)","type":{"title":"General","id":3886},"teaser":"BMF-500, a novel 3 rd generation oral covalent inhibitor of FMS-like tyrosine kinase 3 (FLT3), is the second product candidate discovered and developed by Biomea’s proprietary FUSION™ System to enter the clinic Phase I study (COVALENT-103) of BMF-500 will examine monotherapy safety and efficacy in","language":"en","releaseDate":{"dateUTC":"2023-10-17T12:31:36","date":"2023-10-17T08:31:36","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed with Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia in Phase I Clinical Trial (COVALENT-103)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8226/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-17T12:31:37","lastUpdatedUTC":"2023-10-17T12:31:37"},{"id":8216,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8216","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-2"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for BMF-219 in Type 1 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is a novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure type 1 diabetes The FDA has cleared the initiation of COVALENT-112, a Phase II clinical trial of BMF-219 in adults with type 1 diabetes (T1D).","language":"en","releaseDate":{"dateUTC":"2023-10-05T12:30:41","date":"2023-10-05T08:30:41","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for BMF-219 in Type 1 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8216/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-05T12:30:46","lastUpdatedUTC":"2023-10-05T12:30:46"},{"id":8196,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8196","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-and-health-canada-clearance"},"title":"Biomea Fusion Announces FDA and Health Canada Clearance of the Expansion Cohorts of the Ongoing COVALENT-111 Phase II Study","type":{"title":"General","id":3886},"teaser":"The FDA and Health Canada have cleared the initiation of the expansion portion of COVALENT-111, which will evaluate BMF-219 administered at 100 mg and 200 mg, with dosing durations up to 12 weeks in type 2 diabetes patients The expansion portion will consist of approximately 300 patients and will","language":"en","releaseDate":{"dateUTC":"2023-09-28T12:30:30","date":"2023-09-28T08:30:30","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA and Health Canada Clearance of the Expansion Cohorts of the Ongoing COVALENT-111 Phase II Study","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8196/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-09-28T12:30:34","lastUpdatedUTC":"2023-09-28T12:30:34"},{"id":8171,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8171","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-appointment-juan-pablo-frias-md-chief"},"title":"Biomea Fusion Announces Appointment of Juan Pablo Frías, M.D. as Chief Medical Officer","type":{"title":"General","id":3886},"teaser":"Industry veteran and prominent diabetes clinical development expert to oversee Biomea’s progressing clinical development of novel covalent menin inhibitor BMF-219 in type 2 and type 1 diabetes Steve Morris, M.D., will transition to the role of Chief Development Officer, continuing to lead clinical","language":"en","releaseDate":{"dateUTC":"2023-08-31T12:31:09","date":"2023-08-31T08:31:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Appointment of Juan Pablo Frías, M.D. as Chief Medical Officer","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8171/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-08-31T12:31:11","lastUpdatedUTC":"2023-08-31T12:31:11"},{"id":8141,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8141","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported additional positive clinical data at ADA 83 rd Scientific Sessions from the first two cohorts of patients with type 2 diabetes from ongoing Phase I/II study (COVALENT-111) evaluating BMF-219 as a novel, potentially disease-modifying treatment candidate for patients with type 2 diabetes New","language":"en","releaseDate":{"dateUTC":"2023-07-31T20:01:57","date":"2023-07-31T16:01:57","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8141/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-07-31T20:02:01","lastUpdatedUTC":"2023-07-31T20:02:01"},{"id":8126,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8126","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/bmf-219-induces-complete-responses-target-acute-myeloid-leukemia"},"title":"BMF-219 Induces Complete Responses in Target Acute Myeloid Leukemia (AML) Patient Population","type":{"title":"General","id":3886},"teaser":"Initial topline data from COVALENT-101 trial revealed 2 complete responses (CRs) out of 5 relapsed/refractory AML patients carrying menin-dependent mutations treated at Dose Level 4 Dose Level 4 exposure correlates with initial activity seen in BMF-219’s pre-clinical studies Safety profile of","language":"en","releaseDate":{"dateUTC":"2023-07-24T13:00:45","date":"2023-07-24T09:00:45","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"BMF-219 Induces Complete Responses in Target Acute Myeloid Leukemia (AML) Patient Population","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8126/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-07-24T13:00:52","lastUpdatedUTC":"2023-07-24T13:00:52"},{"id":8076,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8076","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-positive-clinical-data-initial-cohorts"},"title":"Biomea Fusion Presents Positive Clinical Data from the Initial Cohorts of the Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes Mellitus at the American Diabetes Association (ADA) 83rd Scientific Sessions; 100 mg Cohort 3 Demonstrated a 90% Response Rate and 70% Maintained or Improved Time in (Normal Glucose) Range, While Off-Treatment","type":{"title":"General","id":3886},"teaser":"Eight weeks after completing treatment with BMF-219, patients with type 2 diabetes (T2D) showed an increase of C-peptide and an improvement of HOMA-B, measured during oral glucose tolerance testing (OGTT), supporting improved beta cell function for these patients Observations of durable and","language":"en","releaseDate":{"dateUTC":"2023-06-24T01:55:00","date":"2023-06-23T21:55:00","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Positive Clinical Data from the Initial Cohorts of the Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes Mellitus at the American Diabetes Association (ADA) 83rd Scientific Sessions; 100 mg Cohort 3 Demonstrated a 90% Response Rate and 70% Maintained or Improved Time in (Normal Glucose) Range, While Off-Treatment","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8076/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-06-24T01:55:04","lastUpdatedUTC":"2023-06-24T02:30:18"},{"id":8066,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8066","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-late-breaking-data-ongoing-phase-ii-0"},"title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes at ADA 2023","type":{"title":"General","id":3886},"teaser":"New clinical data from COVALENT-111 will be unveiled during a late-breaking poster presentation at ADA’s Scientific Sessions BMF-219, an orally delivered novel covalent menin inhibitor, is designed to regenerate, preserve, and reactivate healthy, insulin-producing beta cells Biomea to hold","language":"en","releaseDate":{"dateUTC":"2023-06-20T20:30:48","date":"2023-06-20T16:30:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes at ADA 2023","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8066/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-06-20T20:30:54","lastUpdatedUTC":"2023-06-20T20:30:54"},{"id":7976,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7976","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported initial positive clinical data from first two cohorts of Phase II of ongoing Phase I/II study (COVALENT-111) of BMF-219, Biomea’s lead investigational, orally administered covalent menin inhibitor, as a novel, potentially disease-modifying treatment for patients with type 2 diabetes","language":"en","releaseDate":{"dateUTC":"2023-05-02T20:01:50","date":"2023-05-02T16:01:50","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7976/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-05-02T20:01:57","lastUpdatedUTC":"2023-05-02T20:01:57"},{"id":7961,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7961","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-1"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"BMF-500, a novel 3 rd generation covalent inhibitor of fms-like tyrosine kinase 3 (FLT3), is the second investigational compound, discovered and developed by Biomea’s FUSION™ System, to advance to the clinic. Phase I study (COVALENT-103) of BMF-500 will examine its safety and efficacy in patients","language":"en","releaseDate":{"dateUTC":"2023-05-01T12:31:22","date":"2023-05-01T08:31:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7961/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-05-01T12:31:33","lastUpdatedUTC":"2023-05-01T12:31:33"},{"id":7921,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7921","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-late-breaking-data-ongoing-phase-ii-trial"},"title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes, at the ADA 83rd Scientific Sessions 2023 in June","type":{"title":"General","id":3886},"teaser":"New clinical data from COVALENT-111 will be featured during the Late-Breaking Poster Presentations at the Scientific Sessions Biomea to hold an investor and KOL event during the Scientific Sessions REDWOOD CITY, Calif., April 19, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2023-04-19T12:30:54","date":"2023-04-19T08:30:54","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes, at the ADA 83rd Scientific Sessions 2023 in June","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7921/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-19T12:31:00","lastUpdatedUTC":"2023-04-19T12:31:00"},{"id":7906,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7906","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-two-preclinical-posters-114th-aacr-annual"},"title":"Biomea Fusion To Present Two Preclinical Posters at the 114th AACR Annual Meeting","type":{"title":"General","id":3886},"teaser":"Abstract 473 highlights the dose dependent reduction of menin target genes in ex vivo chronic lymphocytic leukemia (CLL) patient samples treated with BMF-219, an investigational covalent inhibitor. BMF-219 showed greater potency and ability to achieve >98% growth inhibition in these CLL patient","language":"en","releaseDate":{"dateUTC":"2023-04-13T20:30:31","date":"2023-04-13T16:30:31","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion To Present Two Preclinical Posters at the 114th AACR Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7906/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-13T20:30:35","lastUpdatedUTC":"2023-04-13T20:30:35"},{"id":7896,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7896","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-closing-upsized-public-offering-and-full"},"title":"Biomea Fusion Announces Closing of Upsized Public Offering and Full Exercise of the Underwriters’ Option to Purchase Additional Shares","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., April 13, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically","language":"en","releaseDate":{"dateUTC":"2023-04-13T13:01:10","date":"2023-04-13T09:01:10","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Closing of Upsized Public Offering and Full Exercise of the Underwriters’ Option to Purchase Additional Shares","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7896/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-13T13:01:19","lastUpdatedUTC":"2023-04-13T13:01:19"},{"id":7841,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7841","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-pricing-upsized-public-offering-common"},"title":"Biomea Fusion Announces Pricing of Upsized Public Offering of Common Stock","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 29, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-30T03:15:46","date":"2023-03-29T23:15:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Pricing of Upsized Public Offering of Common Stock","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7841/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-30T03:15:55","lastUpdatedUTC":"2023-03-30T03:15:55"},{"id":7831,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7831","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-proposed-public-offering-common-stock"},"title":"Biomea Fusion Announces Proposed Public Offering of Common Stock","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 29, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-29T10:30:50","date":"2023-03-29T06:30:50","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Proposed Public Offering of Common Stock","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7831/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-29T10:30:58","lastUpdatedUTC":"2023-03-29T10:30:58"},{"id":7806,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7806","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2022"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2022 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Expanded clinical development footprint of BMF-219, the company’s lead investigational, orally administered, covalent menin inhibitor, to eight liquid and solid tumor indications and type 2 diabetes across three ongoing clinical trials COVALENT-101 (Phase I study) enrolling four liquid tumor","language":"en","releaseDate":{"dateUTC":"2023-03-28T20:02:28","date":"2023-03-28T16:02:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2022 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7806/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-28T20:02:30","lastUpdatedUTC":"2023-03-28T20:02:30"},{"id":7796,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7796","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-positive-data-initial-cohorts-ongoing"},"title":"Biomea Fusion Announces Positive Data from Initial Cohorts of Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes; 100 mg Cohort 3 Demonstrated an 89% Response Rate and 1% Median Reduction in HbA1c at Day 28","type":{"title":"General","id":3886},"teaser":"In Cohort 3, after 4 weeks of once-daily 100 mg dosing with the investigational, oral covalent menin inhibitor, BMF-219, 89% of patients achieved a reduction in A1c, 78% of patients achieved at least a 0.5% reduction in A1c, and 56% achieved at least a 1% reduction in A1c.","language":"en","releaseDate":{"dateUTC":"2023-03-28T12:01:22","date":"2023-03-28T08:01:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Positive Data from Initial Cohorts of Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes; 100 mg Cohort 3 Demonstrated an 89% Response Rate and 1% Median Reduction in HbA1c at Day 28","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7796/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-28T12:01:31","lastUpdatedUTC":"2023-03-28T12:01:31"},{"id":7781,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7781","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-and-webcast-discuss-initial"},"title":"Biomea Fusion to Host Conference Call and Webcast to Discuss Initial Phase II Clinical Data for BMF-219 in Subjects with Type 2 Diabetes on March 28th, 2023 at 8:30 a.m. ET","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 23, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-23T12:01:38","date":"2023-03-23T08:01:38","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call and Webcast to Discuss Initial Phase II Clinical Data for BMF-219 in Subjects with Type 2 Diabetes on March 28th, 2023 at 8:30 a.m. ET","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7781/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-23T12:01:50","lastUpdatedUTC":"2023-03-27T17:59:34"},{"id":7741,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7741","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-upcoming-investor-events"},"title":"Biomea Fusion To Participate In Upcoming Investor Events","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 02, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic diseases, today announced that","language":"en","releaseDate":{"dateUTC":"2023-03-02T13:31:37","date":"2023-03-02T08:31:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion To Participate In Upcoming Investor Events","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7741/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-02T13:31:40","lastUpdatedUTC":"2023-03-02T13:31:40"},{"id":7686,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7686","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-doses-first-patient-phase-iib-clinical-trial"},"title":"Biomea Fusion Doses First Patient in Phase I/Ib Clinical Trial (COVALENT-102) of BMF-219 in KRAS Mutant Solid Tumors","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first menin inhibitor to be clinically studied in patients with KRAS-mutated non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC) A pan-KRAS inhibitor targeting multiple KRAS mutations (including G12C, G12D and G12R, among others)","language":"en","releaseDate":{"dateUTC":"2023-01-17T13:02:21","date":"2023-01-17T08:02:21","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Doses First Patient in Phase I/Ib Clinical Trial (COVALENT-102) of BMF-219 in KRAS Mutant Solid Tumors","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7686/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-17T13:02:32","lastUpdatedUTC":"2023-01-17T13:02:32"},{"id":7651,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7651","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-41st-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at 41st Annual J.P. Morgan Healthcare Conference and Highlight 2023 Corporate Milestones","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 09, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2023-01-09T13:01:27","date":"2023-01-09T08:01:27","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at 41st Annual J.P. Morgan Healthcare Conference and Highlight 2023 Corporate Milestones","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7651/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-09T13:01:37","lastUpdatedUTC":"2023-01-09T13:01:37"},{"id":7631,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7631","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-patient-type-2-diabetes"},"title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes in the United States in Ongoing Phase I/II (COVALENT-111) Study of BMF-219","type":{"title":"General","id":3886},"teaser":"COVALENT-111, now underway in the US, has completed Phase I and is currently enrolling type 2 diabetes patients in the Phase II randomized, placebo-controlled portion of the trial BMF-219, an orally available covalent menin inhibitor, is being evaluated for its potential to enable the","language":"en","releaseDate":{"dateUTC":"2023-01-04T14:01:17","date":"2023-01-04T09:01:17","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes in the United States in Ongoing Phase I/II (COVALENT-111) Study of BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7631/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-04T14:01:28","lastUpdatedUTC":"2023-01-04T14:01:28"},{"id":7601,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7601","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-0"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent Menin Inhibitor BMF-219 in Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"COVALENT-111, a Phase I/II clinical trial in patients with type 2 diabetes, already underway in Canada, will now activate sites in the US As previously reported, the Phase I portion of COVALENT-111 has been completed, with BMF-219 demonstrating a favorable safety, pharmacokinetics (PK) and","language":"en","releaseDate":{"dateUTC":"2022-12-14T21:30:31","date":"2022-12-14T16:30:31","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent Menin Inhibitor BMF-219 in Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7601/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-12-14T21:30:40","lastUpdatedUTC":"2022-12-14T22:24:20"},{"id":7581,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7581","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-2022-ash-annual-meeting-preclinical-data"},"title":"Biomea Fusion Presents at the 2022 ASH Annual Meeting Preclinical Data on BMF-500 Supporting its Potential as the Most Potent and Selective FLT3 Inhibitor to Date","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third generation covalent FLT3 inhibitor, demonstrated preclinically: Picomolar affinity to activating FLT3 mutations including FLT3-ITD and various tyrosine kinase domain (TKD) mutations Multi-fold higher potency and increased cytotoxicity than commercially available","language":"en","releaseDate":{"dateUTC":"2022-12-12T13:00:14","date":"2022-12-12T08:00:14","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents at the 2022 ASH Annual Meeting Preclinical Data on BMF-500 Supporting its Potential as the Most Potent and Selective FLT3 Inhibitor to Date","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7581/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-12-12T13:00:24","lastUpdatedUTC":"2022-12-12T13:00:24"},{"id":7526,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7526","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-piper-sandler-34th-annual-healthcare"},"title":"Biomea Fusion to Participate in Piper Sandler 34th Annual Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Nov. 11, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”)(Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2022-11-11T13:30:32","date":"2022-11-11T08:30:32","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Piper Sandler 34th Annual Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7526/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-11T13:30:41","lastUpdatedUTC":"2022-11-11T13:30:41"},{"id":7511,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7511","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"Continued to establish Biomea Fusion as the next-generation leader in covalent medicines Expanded clinical development footprint of BMF-219, the company’s lead investigational, orally administered, covalent menin inhibitor, in multiple liquid and solid tumor indications, including first in class","language":"en","releaseDate":{"dateUTC":"2022-11-07T21:28:19","date":"2022-11-07T16:28:19","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7511/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-07T21:28:23","lastUpdatedUTC":"2022-11-07T21:28:23"},{"id":7496,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7496","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-preclinical-data-bmf-500-investigational"},"title":"Biomea Fusion to Present Preclinical Data for BMF-500, an Investigational Oral Covalent FLT3 Inhibitor, at the 64th American Society of Hematology (ASH) Annual Meeting","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third generation covalent FLT3 inhibitor, has demonstrated preclinically that it may be the most potent and selective inhibitor of FLT3 evaluated to date: Greater Cytotoxicity: In acute myeloid leukemia (AML) cell lines, three-hour treatment with BMF-500 followed by","language":"en","releaseDate":{"dateUTC":"2022-11-03T13:20:05","date":"2022-11-03T09:20:05","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Preclinical Data for BMF-500, an Investigational Oral Covalent FLT3 Inhibitor, at the 64th American Society of Hematology (ASH) Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7496/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-03T13:20:11","lastUpdatedUTC":"2022-11-03T13:20:11"},{"id":7481,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7481","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-patient-type-2-diabetes-and"},"title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes and Completion of Phase I Healthy Volunteer Portion of Phase I/II (COVALENT-111) Study of BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first menin inhibitor to reach the clinic for type 2 diabetes. The Phase II portion of COVALENT-111 is designed to examine the capacity of BMF-219 to provide long-term glycemic control by restoring the patient’s pool of beta cells Beta cell loss is a critical component of the","language":"en","releaseDate":{"dateUTC":"2022-10-31T12:01:54","date":"2022-10-31T08:01:54","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes and Completion of Phase I Healthy Volunteer Portion of Phase I/II (COVALENT-111) Study of BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7481/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-31T12:02:05","lastUpdatedUTC":"2022-10-31T12:02:05"},{"id":7471,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7471","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-chronic-lymphocytic"},"title":"Biomea Fusion Announces First Patient Dosed with Chronic Lymphocytic Leukemia (CLL) in COVALENT-101 Trial","type":{"title":"General","id":3886},"teaser":"COVALENT-101 now includes patients with relapsed/refractory (R/R) CLL BMF-219 is the first menin inhibitor in the clinic for CLL Preclinical data presented at ASCO 2022 demonstrated the potency of BMF-219, a covalent menin inhibitor, across varying cytogenetic risk profiles and Rai stages,","language":"en","releaseDate":{"dateUTC":"2022-10-27T12:01:07","date":"2022-10-27T08:01:07","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed with Chronic Lymphocytic Leukemia (CLL) in COVALENT-101 Trial","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7471/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-27T12:01:18","lastUpdatedUTC":"2022-10-27T12:01:18"},{"id":7441,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7441","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/bmf-219-enters-clinic-kras-solid-tumors"},"title":"BMF-219 Enters the Clinic for KRAS Solid Tumors","type":{"title":"General","id":3886},"teaser":"Biomea Fusion announces FDA clearance of Investigational New Drug (IND) application for covalent menin inhibitor BMF-219 in KRAS solid tumors. Biomea Fusion will now initiate a Phase I/Ib clinical trial (COVALENT-102) of BMF-219 as a monotherapy in patients who have unresectable, locally advanced,","language":"en","releaseDate":{"dateUTC":"2022-10-14T13:00:34","date":"2022-10-14T09:00:34","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"BMF-219 Enters the Clinic for KRAS Solid Tumors","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7441/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-14T13:00:42","lastUpdatedUTC":"2022-10-14T13:00:42"},{"id":7416,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7416","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-new-translational-data-european"},"title":"Biomea Fusion Presents New Translational Data at the European Association for the Study of Diabetes (EASD) 2022 in Animal Models and Ex-vivo Human Donor Islets Further Supporting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Treatment with BMF-219 led to an increase in beta cell mass in ex-vivo experiments with human donor islets BMF-219 showed improved pancreatic beta-cell function and beta cell area, insulin sensitivity, blood lipid levels, weight decline, and glycemic control in the rat models (ZDF (Zucker Diabetic","language":"en","releaseDate":{"dateUTC":"2022-09-22T13:30:32","date":"2022-09-22T09:30:32","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents New Translational Data at the European Association for the Study of Diabetes (EASD) 2022 in Animal Models and Ex-vivo Human Donor Islets Further Supporting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7416/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-09-22T13:30:41","lastUpdatedUTC":"2022-09-22T13:30:41"},{"id":7406,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7406","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-new-preclinical-data-european-association"},"title":"Biomea Fusion Presents New Preclinical Data at the European Association for the Study of Diabetes (EASD) Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Menin, a transcriptional scaffold protein, regulates pancreatic beta cell homeostasis; inhibiting menin function with BMF-219 increased beta cell function in a preclinical animal model, driving an improvement in glycemic control and insulin sensitivity.","language":"en","releaseDate":{"dateUTC":"2022-09-20T11:30:15","date":"2022-09-20T07:30:15","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents New Preclinical Data at the European Association for the Study of Diabetes (EASD) Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7406/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-09-20T11:30:22","lastUpdatedUTC":"2022-09-20T11:30:22"},{"id":7386,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7386","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-additional-preclinical-data-demonstrating"},"title":"Biomea Fusion Presents Additional Preclinical Data Demonstrating Anti-Tumor Activity and Mechanistic Evidence for BMF-219 in Diffuse Large B-Cell Lymphoma and Multiple Myeloma Models at International Myeloma Society Annual Meeting","type":{"title":"General","id":3886},"teaser":"Data demonstrated robust anti-tumor activity of BMF-219 and mechanistic evidence for novel inhibition of menin protein in preclinical models of Diffuse Large B-cell Lymphoma (DLBCL) and multiple myeloma (MM). BMF-219 displayed single agent potency, surpassing greater than 90% inhibition at","language":"en","releaseDate":{"dateUTC":"2022-08-26T12:30:53","date":"2022-08-26T08:30:53","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Additional Preclinical Data Demonstrating Anti-Tumor Activity and Mechanistic Evidence for BMF-219 in Diffuse Large B-Cell Lymphoma and Multiple Myeloma Models at International Myeloma Society Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7386/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-08-26T12:31:31","lastUpdatedUTC":"2022-08-26T12:31:31"},{"id":7346,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7346","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"Continued to make significant progress advancing BMF-219, the company’s lead investigational orally administered covalent menin inhibitor, in multiple oncology indications COVALENT-101 (Phase I) study is enrolling for patients with Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML),","language":"en","releaseDate":{"dateUTC":"2022-08-01T20:05:35","date":"2022-08-01T16:05:35","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7346/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-08-01T20:05:39","lastUpdatedUTC":"2022-08-01T20:05:39"},{"id":7326,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7326","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-selected-two-oral-presentations-european"},"title":"Biomea Fusion Selected for Two Oral Presentations at the European Association for the Study of Diabetes Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting, Acute Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Biomea to present two oral abstracts across multiple animal models highlighting the ability of BMF-219, a covalent menin inhibitor, to significantly lower HbA1C (approximately two-fold greater reduction than active control, liraglutide) and to increase beta cell function.","language":"en","releaseDate":{"dateUTC":"2022-07-01T12:30:12","date":"2022-07-01T08:30:12","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Selected for Two Oral Presentations at the European Association for the Study of Diabetes Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting, Acute Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7326/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-07-01T12:30:17","lastUpdatedUTC":"2022-07-01T12:30:17"},{"id":7276,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7276","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-multiple-myeloma"},"title":"Biomea Fusion Announces First Patient Dosed in Multiple Myeloma Cohort of COVALENT-101 Trial","type":{"title":"General","id":3886},"teaser":"BMF-219, a covalent menin inhibitor, is the first menin inhibitor administered to patients with relapsed/refractory (R/R) multiple myeloma (MM) Patients with R/R MM and R/R diffuse large B-cell lymphoma (DLBCL) are eligible for enrollment in COVALENT-101 Enrollment continuing for both acute","language":"en","releaseDate":{"dateUTC":"2022-06-22T12:30:34","date":"2022-06-22T08:30:34","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed in Multiple Myeloma Cohort of COVALENT-101 Trial","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7276/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-22T12:30:44","lastUpdatedUTC":"2022-06-22T12:30:44"},{"id":7246,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7246","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-novel-preclinical-data-ada-2022"},"title":"Biomea Fusion Presents Novel Preclinical Data at ADA 2022 Suggesting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Menin acts as a ‘brake’ on beta cell regeneration; inhibiting menin function with BMF-219 may increase beta cell production and function, thereby increasing insulin levels and controlling high glucose levels BMF-219, a covalent menin inhibitor, showed strong, prolonged glycemic control, insulin","language":"en","releaseDate":{"dateUTC":"2022-06-04T15:00:48","date":"2022-06-04T11:00:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Novel Preclinical Data at ADA 2022 Suggesting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7246/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-04T15:00:59","lastUpdatedUTC":"2022-06-04T15:00:59"},{"id":7221,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7221","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-jefferies-healthcare-conference"},"title":"Biomea Fusion to Participate in Jefferies Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 02, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-06-02T22:00:08","date":"2022-06-02T18:00:08","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Jefferies Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7221/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-02T22:00:21","lastUpdatedUTC":"2022-06-02T22:00:21"},{"id":7206,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7206","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-preclinical-data-bmf-219-two-diabetic"},"title":"Biomea Fusion to Present New Preclinical Data on BMF-219 in Two Diabetic Animal Models at ADA 2022","type":{"title":"General","id":3886},"teaser":"Company to host virtual investor R&D event on Monday, June 6, 2022 at 4:05 PM EDT featuring covalent menin inhibitor, BMF-219, as a potential novel treatment for type 2 diabetes REDWOOD CITY, Calif., June 01, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2022-06-01T12:30:46","date":"2022-06-01T08:30:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Preclinical Data on BMF-219 in Two Diabetic Animal Models at ADA 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7206/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-01T12:30:50","lastUpdatedUTC":"2022-06-01T12:30:50"},{"id":7196,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7196","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-preclinical-data-showing-bmf-219s"},"title":"Biomea Fusion to Present New Preclinical Data Showing BMF-219’s Strong Activity in Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) Tumor Models at ASCO 2022","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first investigational menin inhibitor in clinical development to show potential as a therapeutic agent for CLL BMF-219, a covalent menin inhibitor, demonstrated potency across ex vivo CLL tumor models with varying cytogenetic risk profiles and Rai stages, indicating broad activity","language":"en","releaseDate":{"dateUTC":"2022-05-26T22:30:28","date":"2022-05-26T18:30:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Preclinical Data Showing BMF-219’s Strong Activity in Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) Tumor Models at ASCO 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7196/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-26T22:30:31","lastUpdatedUTC":"2022-05-26T22:30:31"},{"id":7181,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7181","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-attend-hc-wainwright-global-investment-conference"},"title":"Biomea Fusion to Attend H.C. Wainwright Global Investment Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., May 20, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-05-20T17:30:31","date":"2022-05-20T13:30:31","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Attend H.C. Wainwright Global Investment Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7181/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-20T17:30:42","lastUpdatedUTC":"2022-05-20T17:30:42"},{"id":7166,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7166","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-ind-candidate-selection-bmf-500"},"title":"Biomea Fusion Announces IND Candidate Selection: BMF-500, a Potential Best-in-Class Oral Covalent Inhibitor of FLT3","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third-generation covalent inhibitor of FLT3, demonstrated picomolar IC 50 values across key FLT3 isoforms, potentially making it the most potent inhibitor of its class Highly selective for FLT3, BMF-500 was observed to avoid other type III receptor tyrosine kinase (RTK)","language":"en","releaseDate":{"dateUTC":"2022-05-19T12:00:39","date":"2022-05-19T08:00:39","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces IND Candidate Selection: BMF-500, a Potential Best-in-Class Oral Covalent Inhibitor of FLT3","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7166/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-19T12:00:48","lastUpdatedUTC":"2022-05-19T12:00:48"},{"id":7146,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7146","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"COVALENT-101 study continues to enroll relapsed/refractory (R/R) acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL) patients and has included R/R diffuse large B-cell lymphoma (DLBCL) and R/R multiple myeloma (MM) patients to the study.","language":"en","releaseDate":{"dateUTC":"2022-05-16T11:30:22","date":"2022-05-16T07:30:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7146/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-16T11:30:30","lastUpdatedUTC":"2022-05-16T11:30:30"},{"id":7131,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7131","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-bank-america-securities-2022"},"title":"Biomea Fusion to Participate in Bank of America Securities 2022 Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., May 09, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-05-09T20:30:13","date":"2022-05-09T16:30:13","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Bank of America Securities 2022 Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7131/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-09T20:30:25","lastUpdatedUTC":"2022-05-09T20:30:25"},{"id":7106,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7106","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-acceptance-multiple-abstracts-2022-asco"},"title":"Biomea Fusion Announces Acceptance of Multiple Abstracts at the 2022 ASCO Annual Meeting","type":{"title":"General","id":3886},"teaser":"Biomea to present preclinical data in chronic lymphocytic leukemia (CLL) and Trial In Progress (TIP) information for its ongoing COVALENT-101 Phase I trial REDWOOD CITY, Calif., April 27, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company","language":"en","releaseDate":{"dateUTC":"2022-04-27T20:00:29","date":"2022-04-27T16:00:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Acceptance of Multiple Abstracts at the 2022 ASCO Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7106/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-04-27T20:00:40","lastUpdatedUTC":"2022-04-27T20:00:40"},{"id":7081,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7081","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-preclinical-data-bmf-219-and-trial"},"title":"Biomea Fusion Reports Preclinical Data on BMF-219 and Trial in Progress Presentations at AACR 2022 Annual Meeting","type":{"title":"General","id":3886},"teaser":"Covalent menin inhibitor BMF-219 showed strong cytotoxic activity as a single agent at similar concentrations across multiple preclinical patient derived (PDX) models ex vivo , including diffuse large B-cell lymphoma (DLBCL), multiple myeloma (MM), colorectal cancer (CRC), non-small cell lung","language":"en","releaseDate":{"dateUTC":"2022-04-08T17:00:28","date":"2022-04-08T13:00:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Preclinical Data on BMF-219 and Trial in Progress Presentations at AACR 2022 Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7081/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-04-08T17:01:20","lastUpdatedUTC":"2022-04-08T17:01:20"},{"id":7066,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7066","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-acceptance-late-breaking-presentation"},"title":"Biomea Fusion Announces Acceptance of Late Breaking Presentation of Lead Menin Inhibitor BMF-219 in Diabetes at ADA 82nd Scientific Sessions 2022","type":{"title":"General","id":3886},"teaser":"Biomea to present additional preclinical data from an in vivo study of BMF-219 in type 2 diabetes REDWOOD CITY, Calif., April 06, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel covalent","language":"en","releaseDate":{"dateUTC":"2022-04-06T21:00:28","date":"2022-04-06T17:00:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Acceptance of Late Breaking Presentation of Lead Menin Inhibitor BMF-219 in Diabetes at ADA 82nd Scientific Sessions 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7066/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-04-06T21:00:53","lastUpdatedUTC":"2022-04-06T21:00:53"},{"id":7031,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7031","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-oppenheimers-32nd-annual-virtual"},"title":"Biomea Fusion to Present at Oppenheimer’s 32nd Annual Virtual Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 15, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible covalent small molecules to treat and improve the lives of patients with genetically defined","language":"en","releaseDate":{"dateUTC":"2022-03-15T12:30:35","date":"2022-03-15T08:30:35","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at Oppenheimer’s 32nd Annual Virtual Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7031/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-03-15T12:31:07","lastUpdatedUTC":"2022-03-15T12:31:07"},{"id":7021,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7021","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-presentation-lead-menin-inhibitor-bmf"},"title":"Biomea Fusion Announces Presentation of Lead Menin Inhibitor BMF-219 in Diabetes at ADA 82nd Scientific Sessions 2022","type":{"title":"General","id":3886},"teaser":"Biomea to present preclinical data from multiple in vivo studies Biomea plans to initiate a Phase I/II clinical trial of BMF-219 in diabetes in the second half of 2022, subject to submission and clearance of an investigational new drug (IND) application REDWOOD CITY, Calif., March 14, 2022 (GLOBE","language":"en","releaseDate":{"dateUTC":"2022-03-14T12:30:35","date":"2022-03-14T08:30:35","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Presentation of Lead Menin Inhibitor BMF-219 in Diabetes at ADA 82nd Scientific Sessions 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7021/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-03-14T12:30:53","lastUpdatedUTC":"2022-03-14T12:30:53"},{"id":7011,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7011","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-upcoming-presentations-preclinical-data"},"title":"Biomea Fusion Announces Upcoming Presentations of Preclinical Data in Diffuse Large B-Cell Lymphoma, Multiple Myeloma, and Several KRAS Mutant Solid Tumors for BMF-219 at AACR Annual Meeting 2022","type":{"title":"General","id":3886},"teaser":"Irreversible covalent menin inhibitor, BMF-219, exhibited high potency and complete growth inhibition in high-grade B-cell lymphoma and multiple myeloma (MM) preclinical patient derived ex vivo models BMF-219 demonstrated high potency in various KRAS-mutant cell lines, as well as potential","language":"en","releaseDate":{"dateUTC":"2022-03-08T23:15:30","date":"2022-03-08T18:15:30","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Upcoming Presentations of Preclinical Data in Diffuse Large B-Cell Lymphoma, Multiple Myeloma, and Several KRAS Mutant Solid Tumors for BMF-219 at AACR Annual Meeting 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7011/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-03-08T23:15:33","lastUpdatedUTC":"2022-03-08T23:15:33"},{"id":6976,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6976","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2021"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2021 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Phase I trial of BMF-219, an irreversible covalent menin inhibitor, is currently underway Plan to initiate trials of BMF-219 in up to seven solid and liquid tumor types and diabetes Plan to announce second clinical candidate in the first half of 2022 Cash, cash equivalents, restricted cash, and","language":"en","releaseDate":{"dateUTC":"2022-02-28T21:05:36","date":"2022-02-28T16:05:36","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2021 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6976/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-02-28T21:05:49","lastUpdatedUTC":"2022-02-28T21:05:49"},{"id":6921,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6921","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-appointment-steve-morris-md-chief"},"title":"Biomea Fusion Announces Appointment of Steve Morris, M.D. as Chief Medical Officer","type":{"title":"General","id":3886},"teaser":"Renowned physician-scientist’s groundbreaking work led to the discovery and characterization of multiple oncogenes, including anaplastic lymphoma kinase (ALK) Dr. Morris will lead clinical development of BMF-219, an irreversible covalent menin inhibitor currently in a Phase I clinical trial, and","language":"en","releaseDate":{"dateUTC":"2022-02-01T11:30:32","date":"2022-02-01T06:30:32","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Appointment of Steve Morris, M.D. as Chief Medical Officer","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6921/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-02-01T11:30:34","lastUpdatedUTC":"2022-02-01T11:30:34"},{"id":6911,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6911","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed"},"title":"Biomea Fusion Announces First Patient Dosed","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first irreversible covalent menin inhibitor to enter the clinic Phase I trial is enrolling patients with relapsed/refractory acute leukemias, including those with MLL1/KMT2A gene rearrangements or NPM1 mutations Phase I trial is expected to include up to 20 clinical sites at leading","language":"en","releaseDate":{"dateUTC":"2022-01-25T13:31:13","date":"2022-01-25T08:31:13","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6911/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-01-25T13:31:30","lastUpdatedUTC":"2022-01-25T13:31:30"},{"id":6876,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6876","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-participation-hc-wainwright-2022"},"title":"Biomea Fusion Announces Participation at the H.C. Wainwright 2022 BioConnect Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 11, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible small molecules to treat and improve the lives of patients with genetically defined","language":"en","releaseDate":{"dateUTC":"2022-01-11T13:30:38","date":"2022-01-11T08:30:38","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Participation at the H.C. Wainwright 2022 BioConnect Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6876/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-01-11T13:30:45","lastUpdatedUTC":"2022-01-11T13:30:45"},{"id":6861,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6861","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-2022-clinical-development-plan-initiate"},"title":"Biomea Fusion Announces 2022 Clinical Development Plan to Initiate Studies in up to Seven Different Tumor Types and in Diabetes for BMF-219","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 10, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible small molecules to treat and improve the lives of patients with genetically defined","language":"en","releaseDate":{"dateUTC":"2022-01-10T13:30:24","date":"2022-01-10T08:30:24","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces 2022 Clinical Development Plan to Initiate Studies in up to Seven Different Tumor Types and in Diabetes for BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6861/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-01-10T13:30:27","lastUpdatedUTC":"2022-01-10T13:30:27"},{"id":6846,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6846","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-releases-pre-clinical-data-bmf-219-diabetes"},"title":"Biomea Fusion Releases Pre-Clinical Data with BMF-219 in Diabetes","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 06, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of irreversible small molecules to treat and improve the lives of patients with genetically defined cancers","language":"en","releaseDate":{"dateUTC":"2022-01-06T13:30:42","date":"2022-01-06T08:30:42","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Releases Pre-Clinical Data with BMF-219 in Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6846/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-01-06T13:30:53","lastUpdatedUTC":"2022-01-06T13:30:53"},{"id":6836,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6836","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-moves-new-headquarters-and-expands-rd-facility"},"title":"Biomea Fusion Moves into New Headquarters and Expands R&D Facility","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 04, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible small molecules to treat and improve the lives of patients with genetically defined","language":"en","releaseDate":{"dateUTC":"2022-01-04T18:30:39","date":"2022-01-04T13:30:39","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Moves into New Headquarters and Expands R&D Facility","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6836/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-01-04T18:30:44","lastUpdatedUTC":"2022-01-04T18:30:44"},{"id":6826,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6826","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-40th-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at the 40th Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Dec. 21, 2021 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel irreversible small molecules to treat and improve the lives of patients with genetically defined","language":"en","releaseDate":{"dateUTC":"2021-12-21T13:01:21","date":"2021-12-21T08:01:21","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 40th Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/6826/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2021-12-21T13:01:28","lastUpdatedUTC":"2021-12-21T13:01:28"}],"error":null};
var latestArticle = prData.data[0];
document.addEventListener("DOMContentLoaded", function() {
// console.log(document.getElementsByClassName('njt-nofi-text'));
document.getElementsByClassName('njt-nofi-text')[0].innerHTML = '
' + latestArticle.title + '';
// console.log(latestArticle);
});