This digital illustration highlights beta cells (green) – responsible for the insulin production – and alpha cells (red) responsible for glucagon production. The nuclei of both cells is shown in blue. – Image credit: Ge Li / Waterland lab / Environmental Epigenetics, 2019.
Diabetes & Obesity Pipeline
The following table summarizes our product candidate pipeline. We own full worldwide development and commercialization rights to all of our program


Menin, Diabetes & Biomea Fusion
About Diabetes
Diabetes is a chronic health condition that affects how the body metabolizes nutrients. It results in too much glucose (sugar) in the bloodstream. Over time, this can cause serious health problems by damaging vital tissues and organs. Most people with diabetes have a shorter life expectancy than people without this disease. The Centers for Disease Control (CDC) estimates about 2 in 5 adults in the US will develop diabetes during their lifetime, more than 37 million Americans of all ages (about 11% of the US population) have diabetes today and an additional 96 million adults (more than 1 in 3) have prediabetes, glucose levels that are higher than normal but not high enough to be classified as diabetes. Diabetes is also one of the largest economic burdens on the health care system, with $1 out of every $4 in US health care costs being spent on caring for people with diabetes.
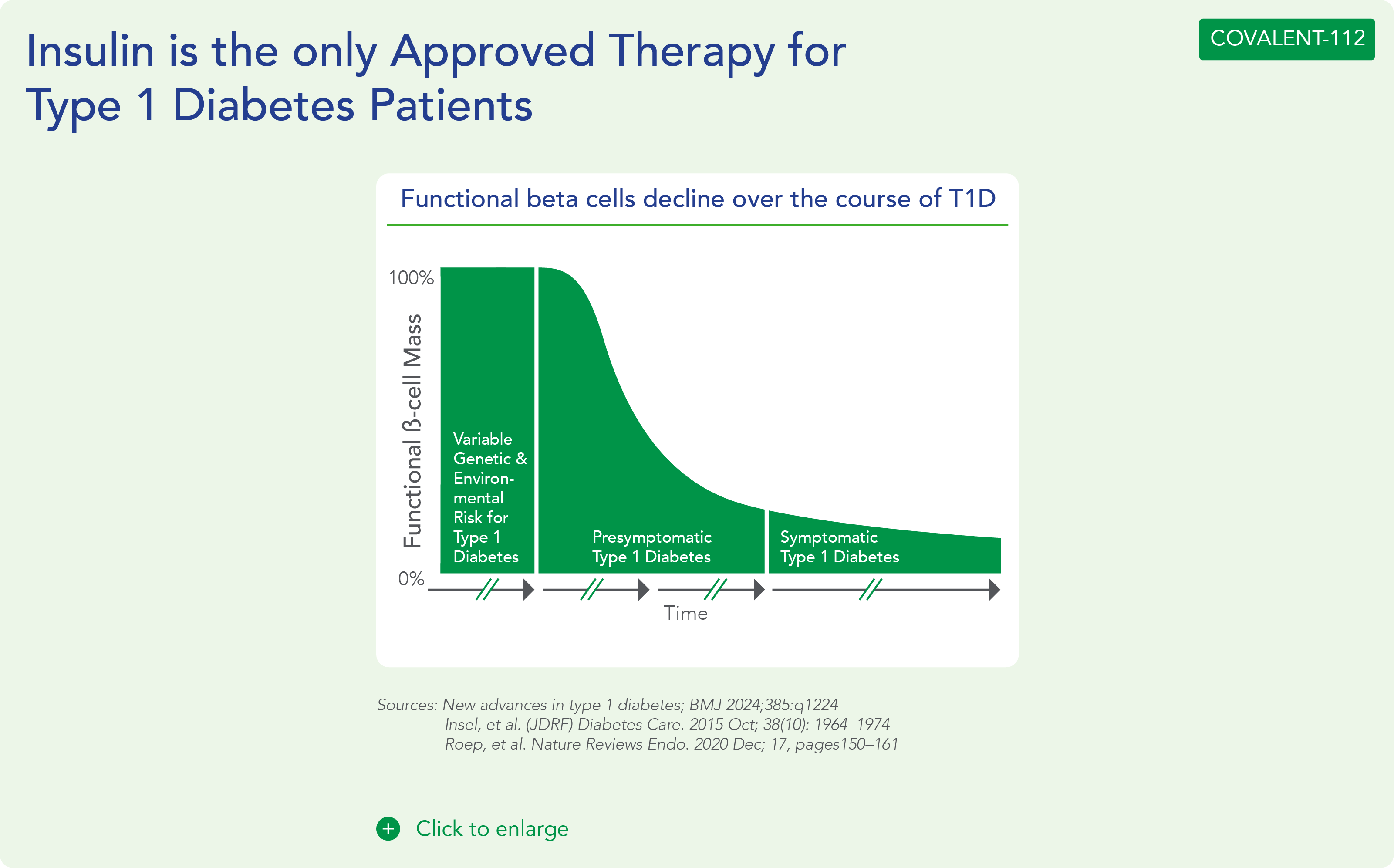
Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta-cell mass and function are diminished, leading to insufficient insulin secretion and elevated glucose levels (hyperglycemia).
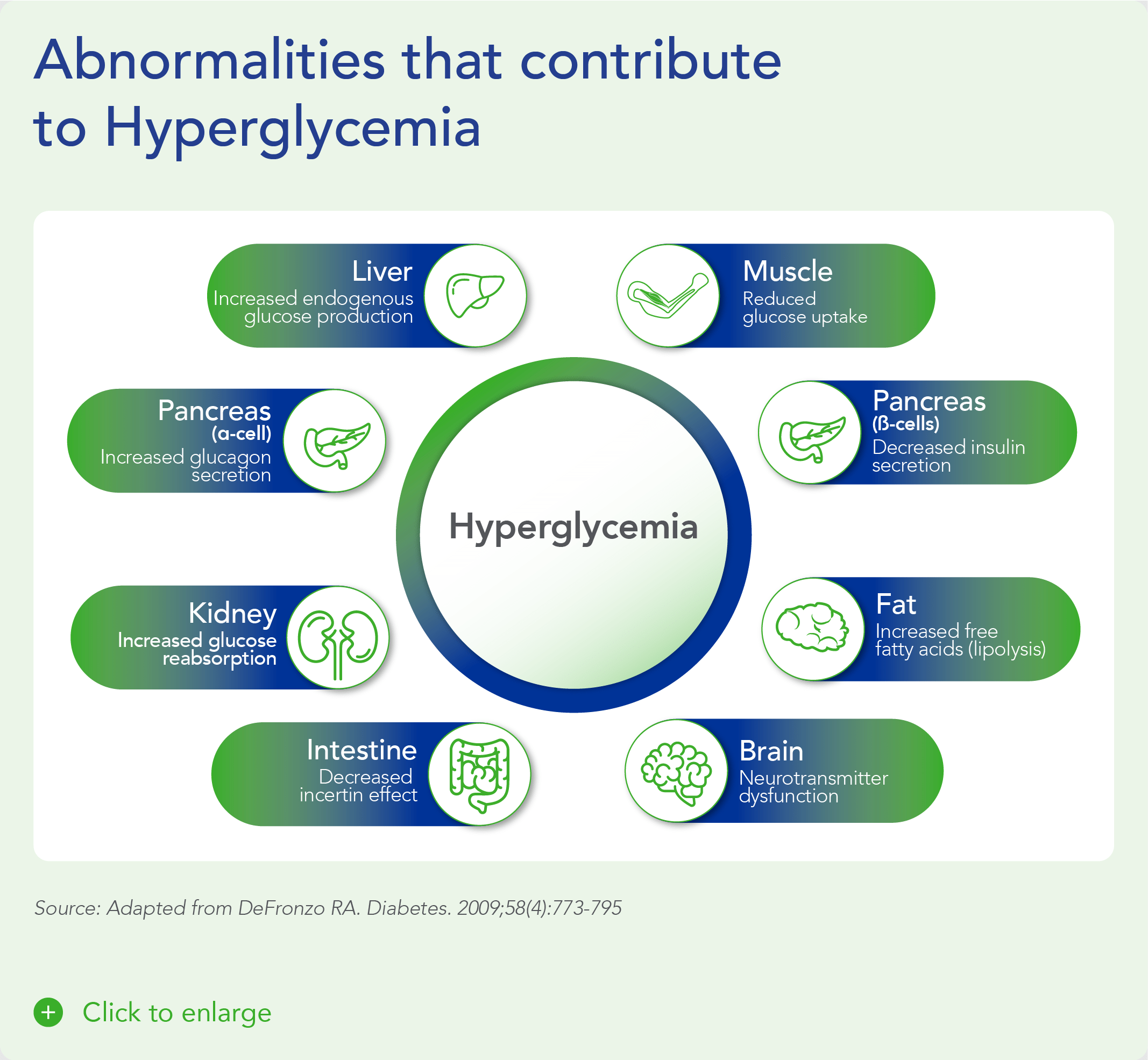
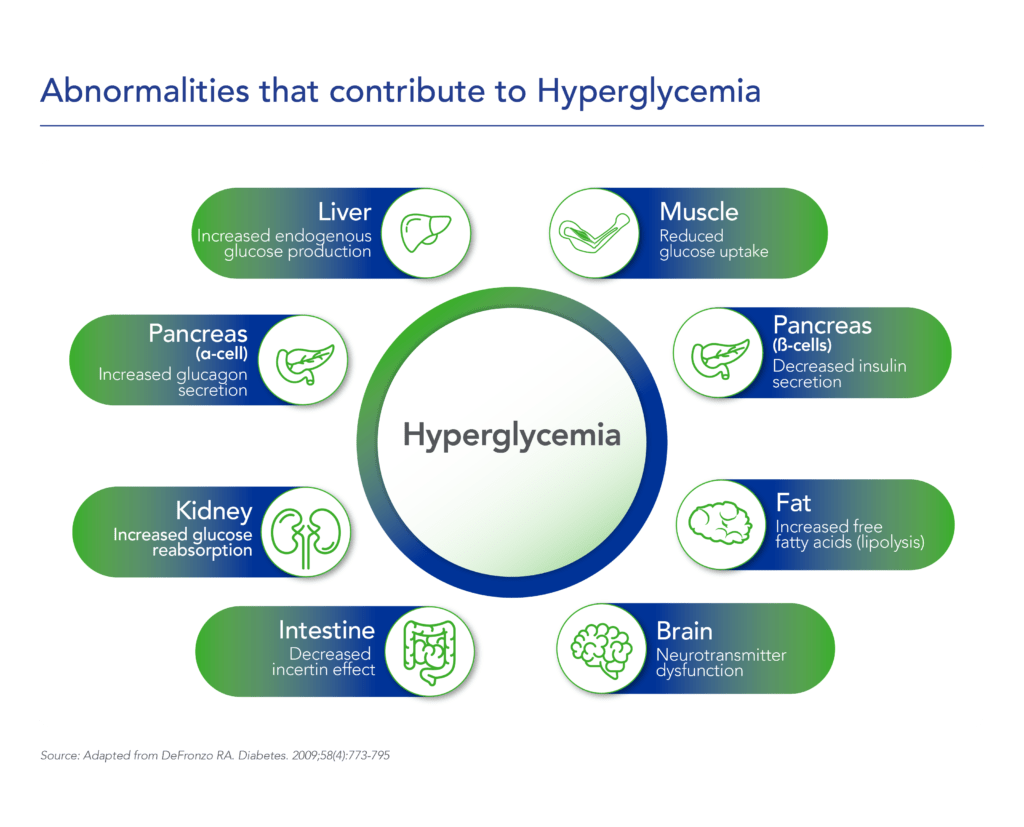
There are two major types of diabetes: type 1 diabetes (T1D) and type 2 diabetes (T2D). T1D is caused by an autoimmune reaction (the body “attacks itself” by mistake). This reaction destroys insulin-producing beta cells in the pancreas, stopping the body’s ability to produce and secrete insulin. Approximately 5-10% of the people who have diabetes have T1D. With T2D, the body doesn’t use insulin well and can’t keep blood sugar at normal levels. About 90-95% of people with diabetes have T2D. Loss of functional beta-cell mass is a core component of the natural history in both T1D and T2D.
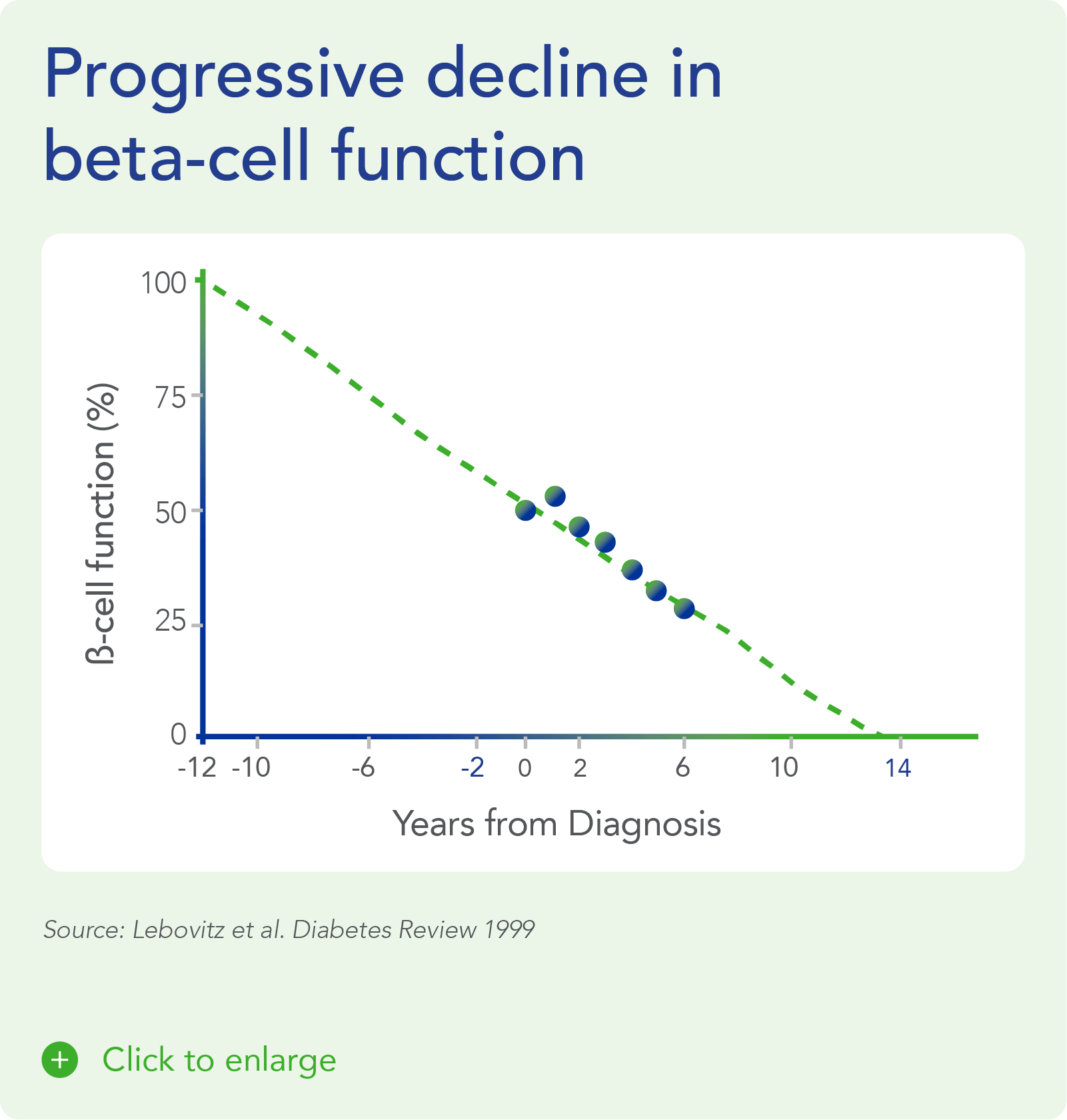
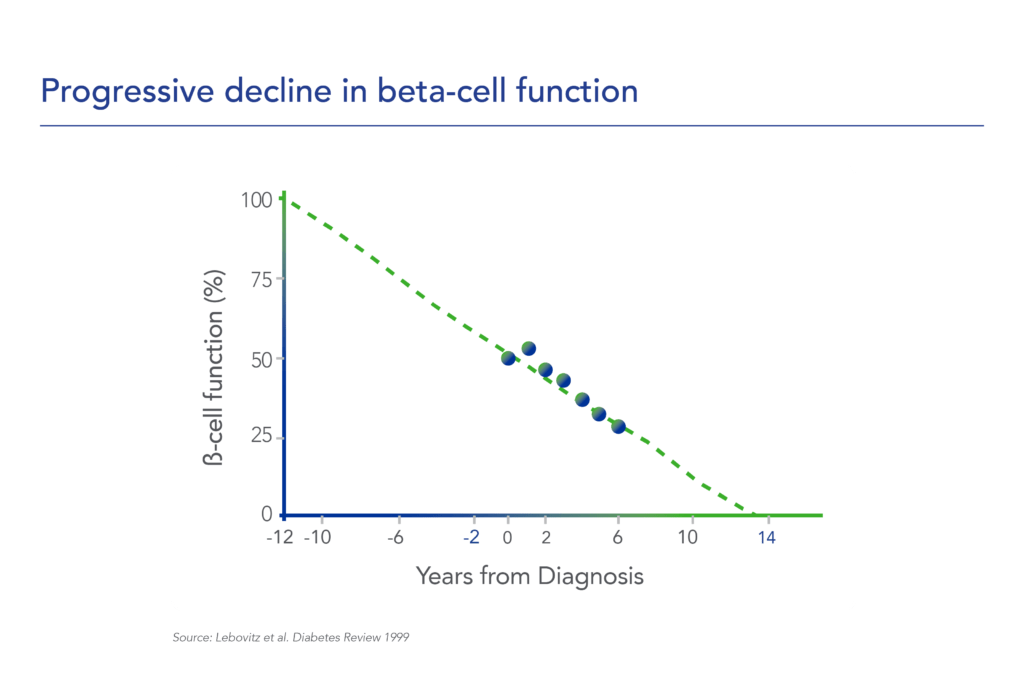
Despite significant advances in pharmacotherapy and diabetes-related devices over the past 2 decades, it is estimated that approximately 50% of persons with diabetes in the US do not have adequate glucose control, as defined by a glycated hemoglobin (HbA1C) of 7% or less. One important reason is that current agents for the management of T1D and T2D do not address the root cause of diabetes – a progressive decline in beta-cell mass and function.





Menin in Diabetes
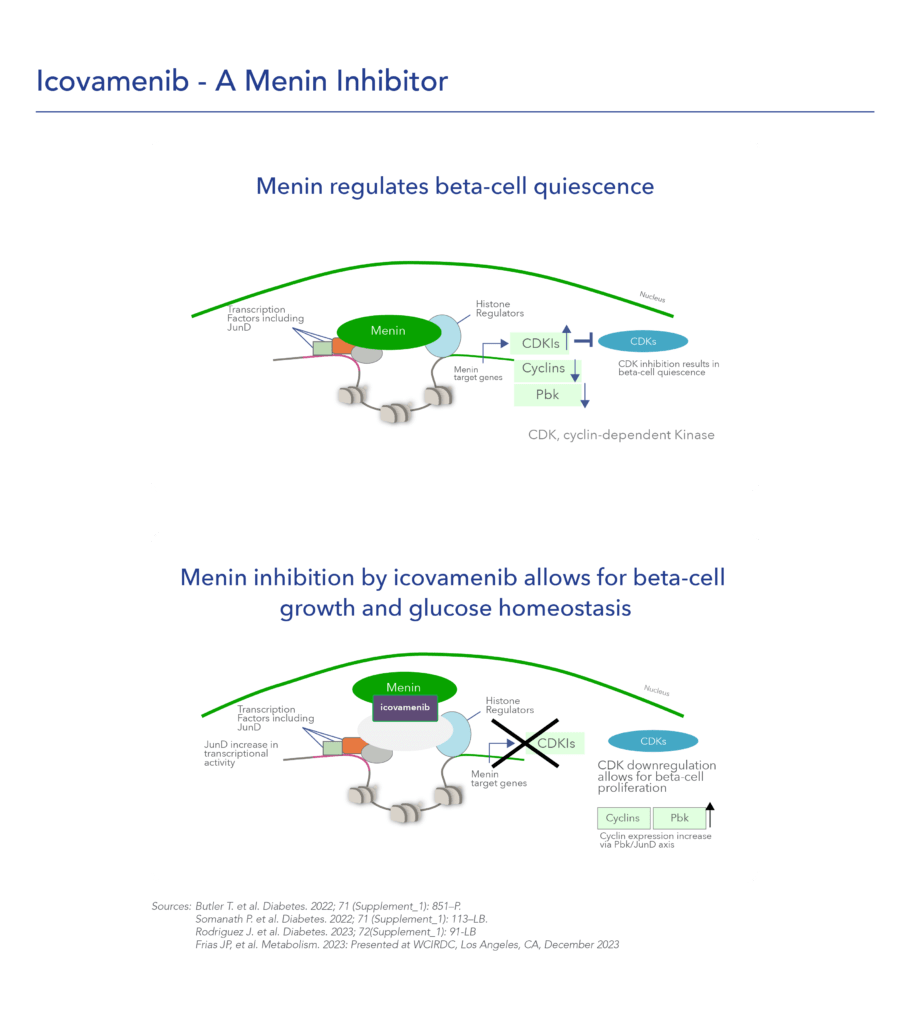
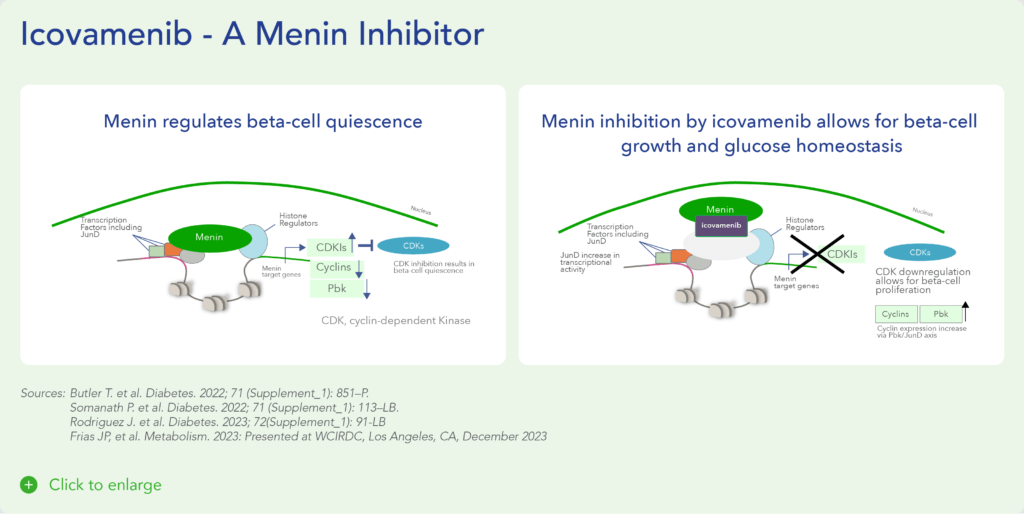
Menin is a transcriptional scaffold protein that controls the expression and activity of proteins that regulate beta-cell proliferation. Menin is thought to control beta-cell proliferation and mass by acting as a brake on beta-cell turnover / beta-cell growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells, which could be a disease-modifying approach to treat diabetes.
Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta cell mass and function are diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on beta-cell turnover and growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells.

Normal Role Of Menin In Beta Cell Regulation
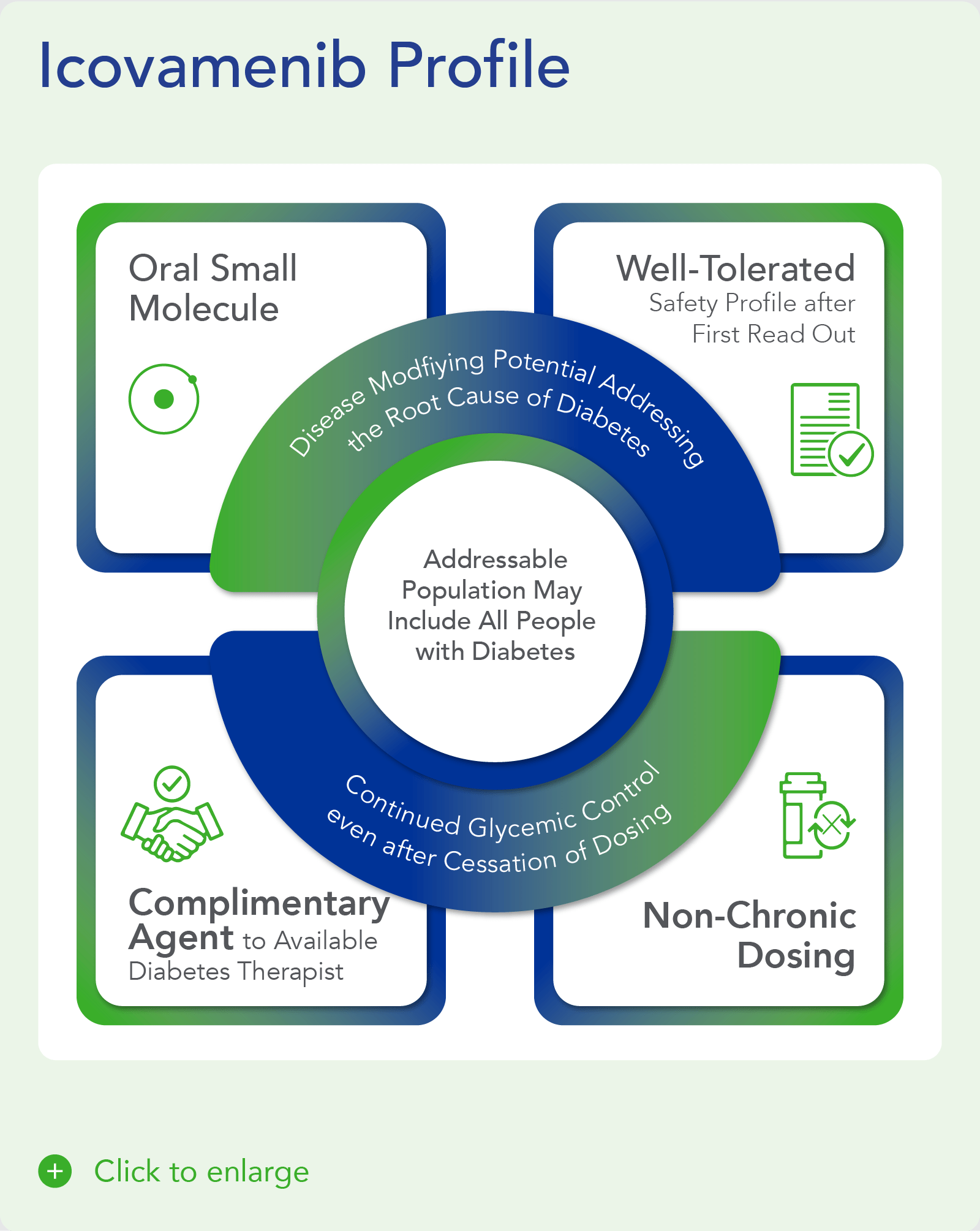
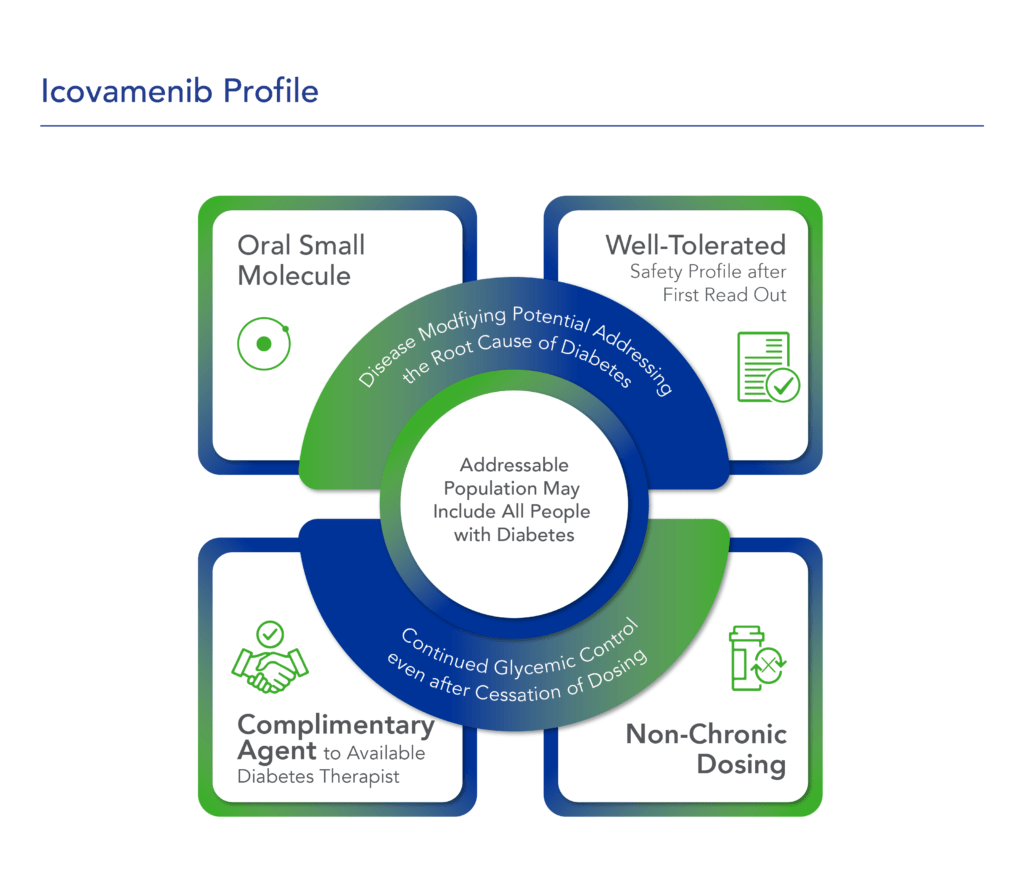
Icovamenib – Potential Mechanism of Action
Icovamenib, is an orally bioavailable, potent and selective covalent inhibitor of menin, built using Biomea Fusion’s FUSION™ System. The molecule is designed to regenerate insulin-producing beta cells with the aim to cure diabetes.
Loss of functional beta cell mass is a core component of the natural history in both types of diabetes — type 1 diabetes (mediated by autoimmune beta cell death) and type 2 diabetes (mediated by metabolic dysfunction leading to beta cell death). Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta cell mass and function are diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on beta-cell turnover and growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells.

How Does Icovameninb Work?

How Does Icovameninb Work?
Icovamenib’s proposed mechanism of action in diabetes is to enable the proliferation, preservation, and reactivation of a patient’s own healthy, functional, insulin-producing beta cells. As the potentially first disease-modifying therapy for type 1 and type 2 diabetes, icovamenib could be an important addition and complement to the diabetes treatment landscape, if approved.




Preclinical Development
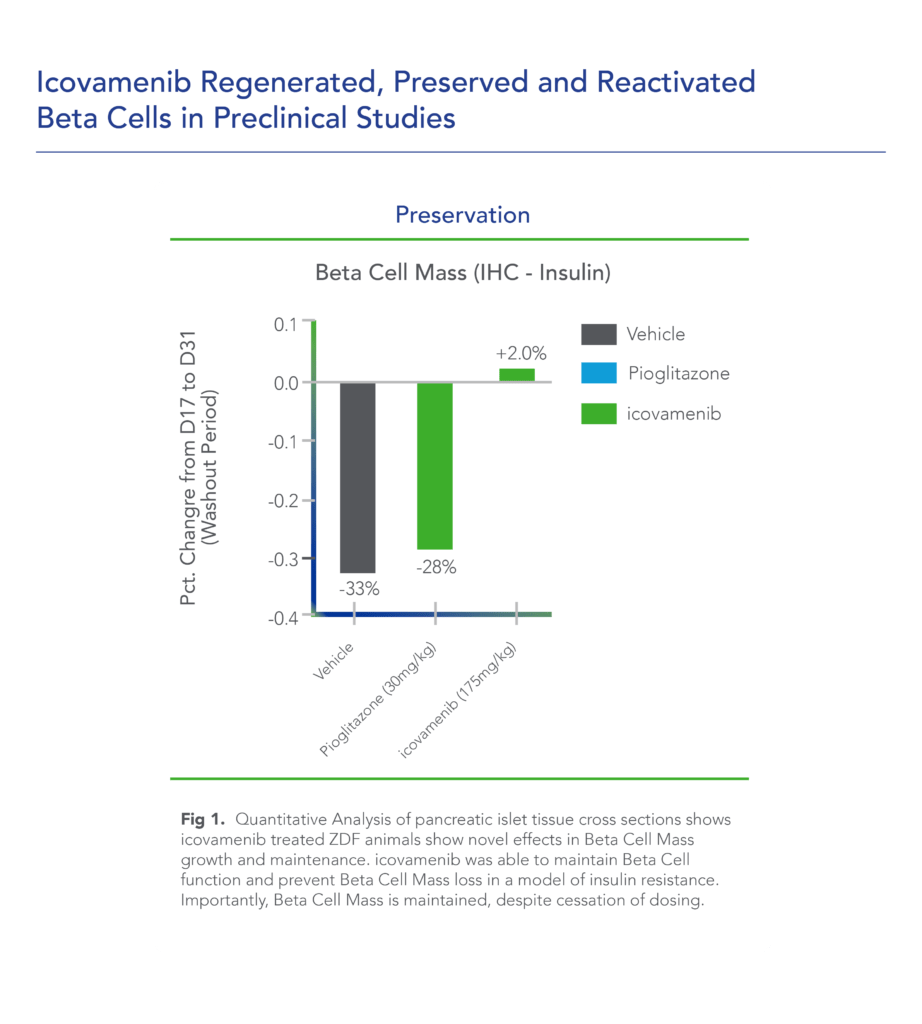
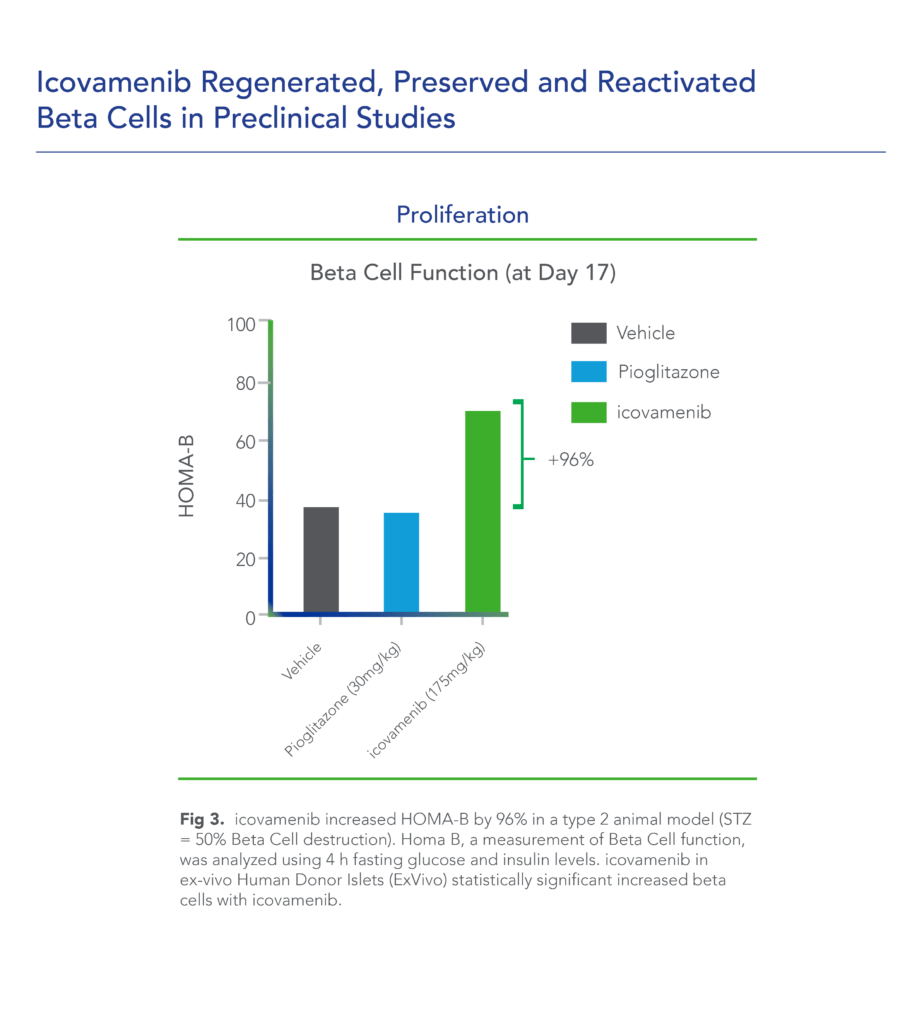
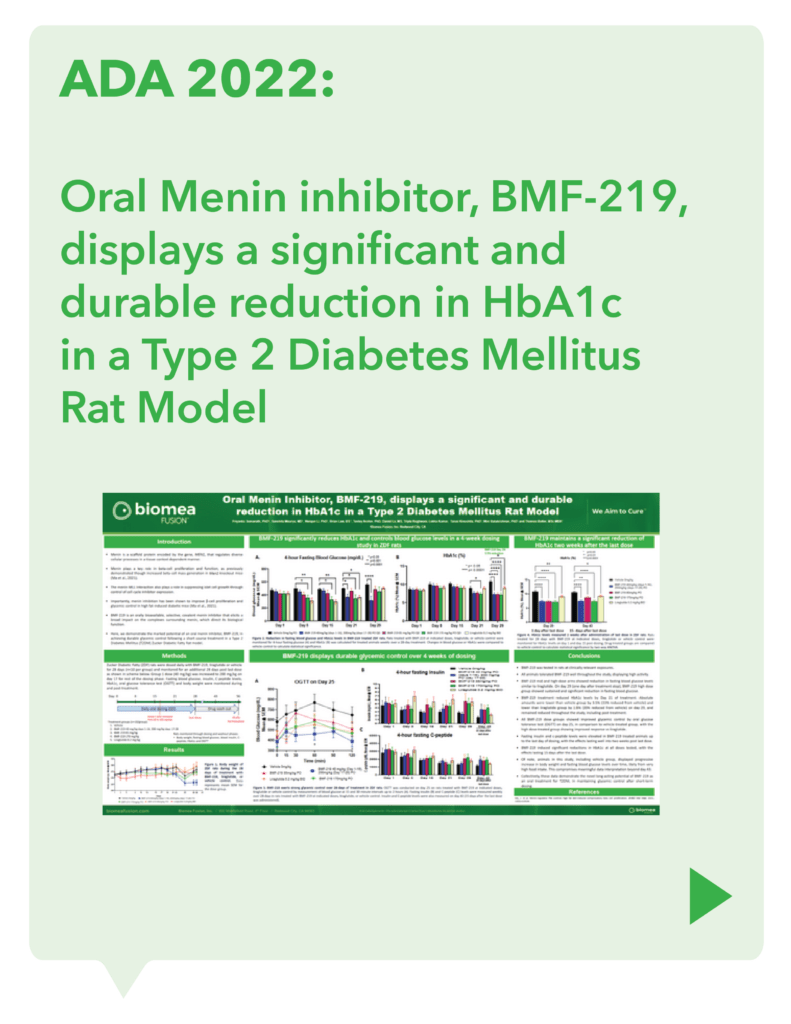
Biomea conducted two diabetes animal experiments to measure the potential impact of icovamenib for the treatment of type 2 diabetes: the Zucker Diabetic Fatty (ZDF) rat, a widely studied model of obesity and insulin resistance in rats, and the Streptozotocin (STZ)-induced diabetic rat, another frequently used model in which diabetes is induced using a chemical (STZ) that selectively destroys beta cells. In both models, icovamenib was able to normalize glucose levels in the majority of animals after just 2 weeks of treatment. Notably, the majority of the effect was maintained even after the two week icovamenib treatment period. (Butler et al, EASD 2022; Somanath et al. EASD 2022)
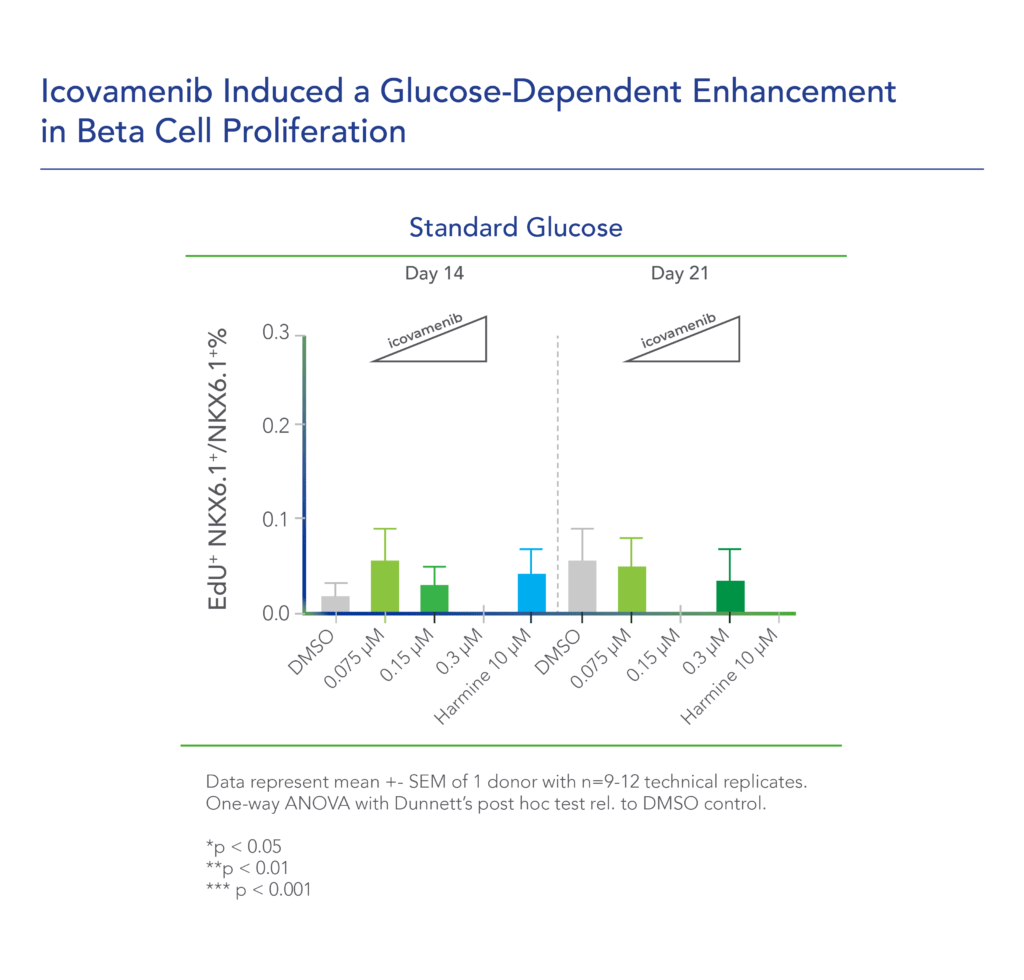
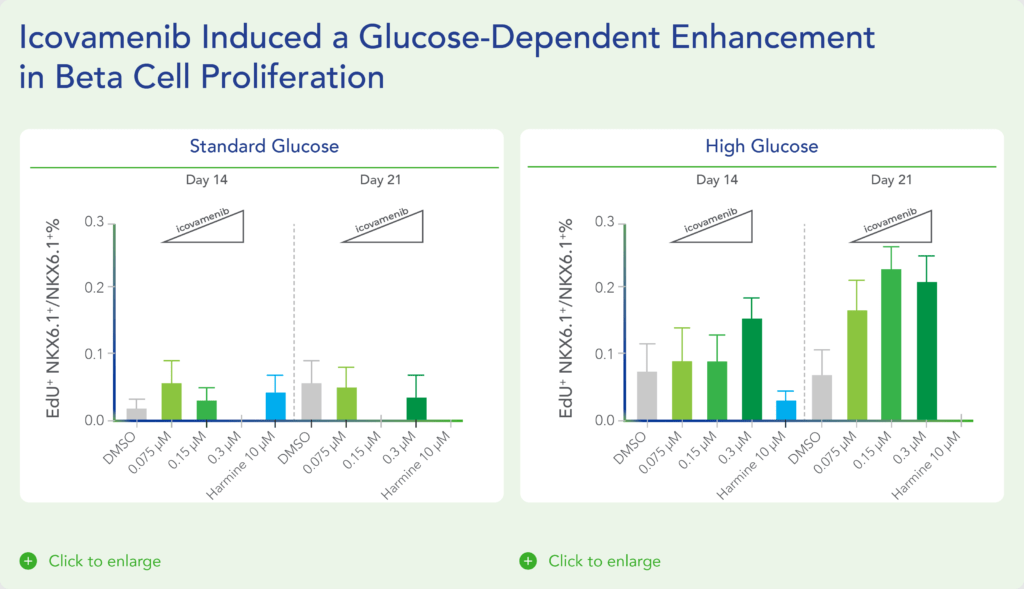
In additional preclinical studies, ex vivo human pancreatic islet cultures were performed to assess the effect of icovamenib. Dependent on the concentration and duration of exposure, icovamenib was shown to increase the beta-cell mass and function, promoting glucose-dependent proliferation and enhancement of insulin production. Beta cell proliferation was observed only under elevated glucose conditions, which mimic diabetic levels, and with continuous drug exposure. icovamenib was demonstrated to upregulate the expression of key proteins involved in cell-cycle regulation such as PbK and CCNA2 (Cyclin A2) in a glucose-dependent fashion. (Kulkarni et al. WCIRDC 2023)
Clinical Development
About Type 2 Diabetes
According to the International Diabetes Foundation, worldwide over 500 million adults have diabetes growing to over 600 million within this decade. In the United States alone, approximately 35 million adults have diabetes and over 90 million adults have pre-diabetes. Type 2 Diabetes is one of the largest economic burdens on the US health care system and the 7th leading cause of death in the US (Source: Diabetes.org) The prevalence of type 2 diabetes is increasing rapidly, particularly among younger age groups. Estimates suggest that people with type 2 diabetes die, on average, 6 years earlier than people without diabetes (Source: The Lancet. 2023 Sept). Despite increased research efforts over the past decades and with over 60 approved therapies to date, diabetes remains unresolved for almost 50% of patients on standard of care (source Clin Diabetes. 2020 Jul).
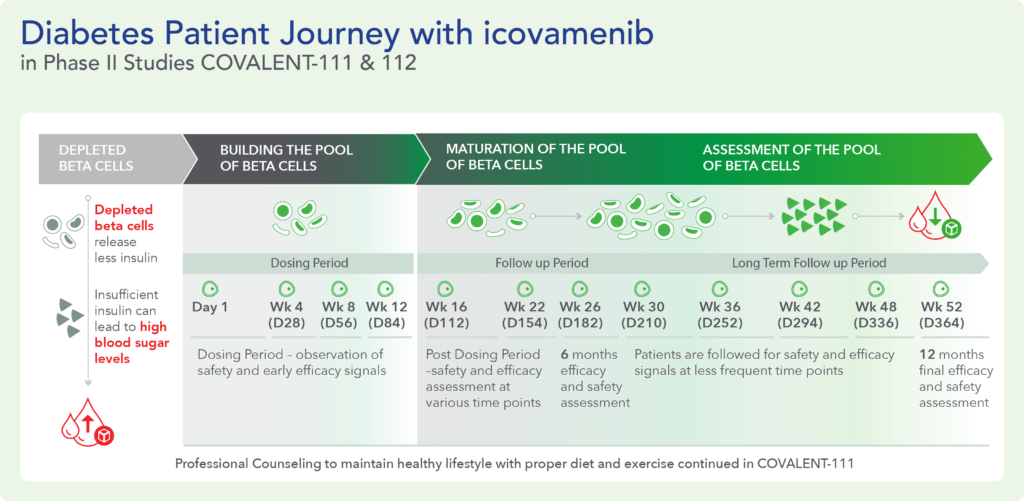
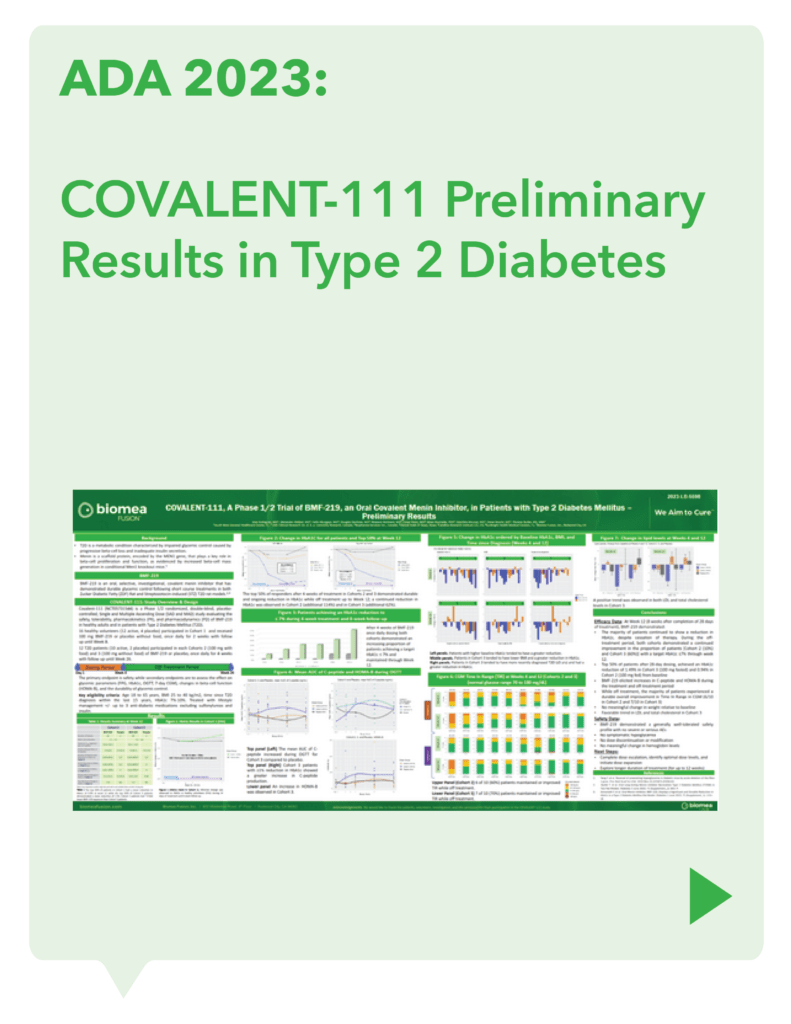
COVALENT-111 (Type 2 Diabetes)
Clinical Design and Results
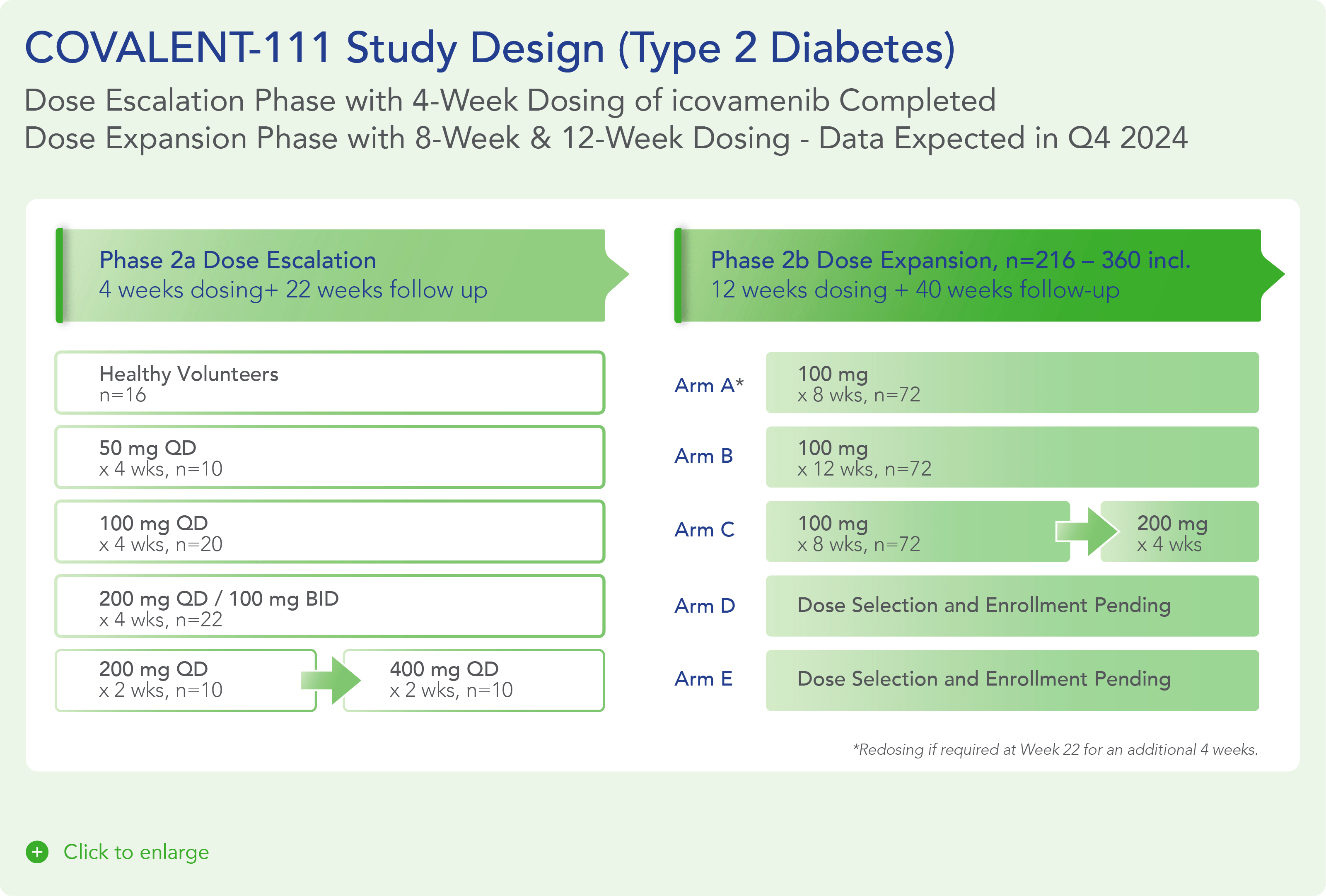
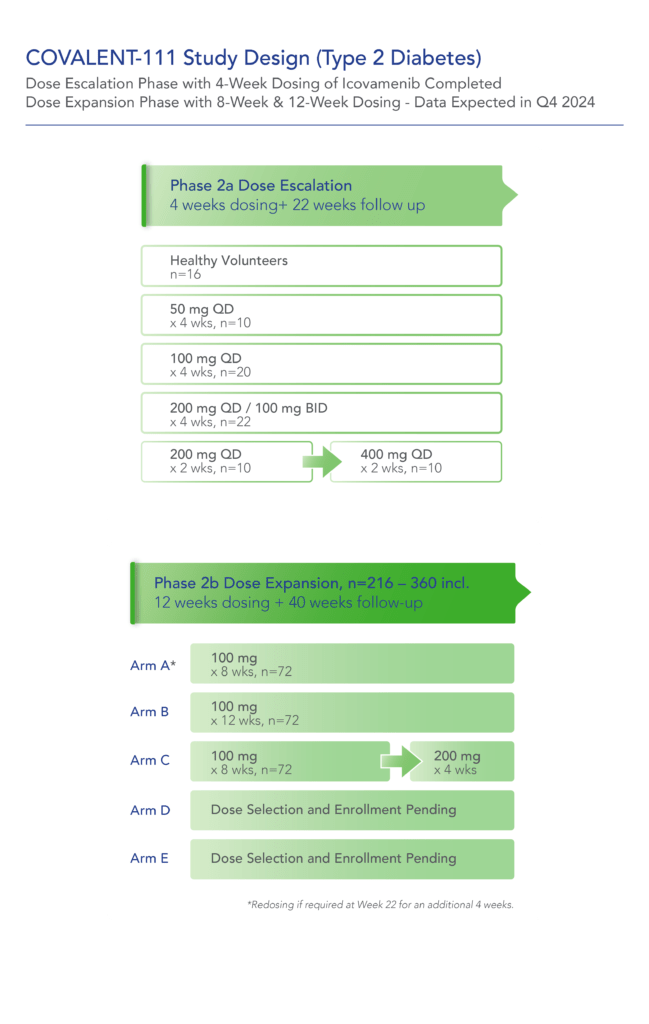
COVALENT-111 is a multi-site, randomized, double-blind, placebo-controlled Phase I/II study. In the completed Phase I portion of the trial, healthy volunteers were enrolled in single ascending dose cohorts to evaluate safety at the prospective dosing levels for patients with type 2 diabetes. Phase II consists of multiple ascending dose cohorts and includes adult patients with type 2 diabetes uncontrolled by standard of care medicines. The dose escalation phase evaluated icovamenib dosed over 4 weeks with 22 weeks follow-up off treatment. Following the Escalation Phase of COVALENT-111, the study has advanced into an Expansion Phase (n>200) consisting of multiple cohorts dosing type 2 diabetes patients for longer dose durations. The first three arms (A, B, C) of the expansion phase are evaluating icovamenib dosed over 8 and 12 weeks at 100 mg and 200 mg with up to 40 weeks of follow-up off treatment. Additional information about the Phase I/II clinical trial of icovamenib in type 2 diabetes can be found at ClinicalTrials.gov using the identifier NCT05731544.

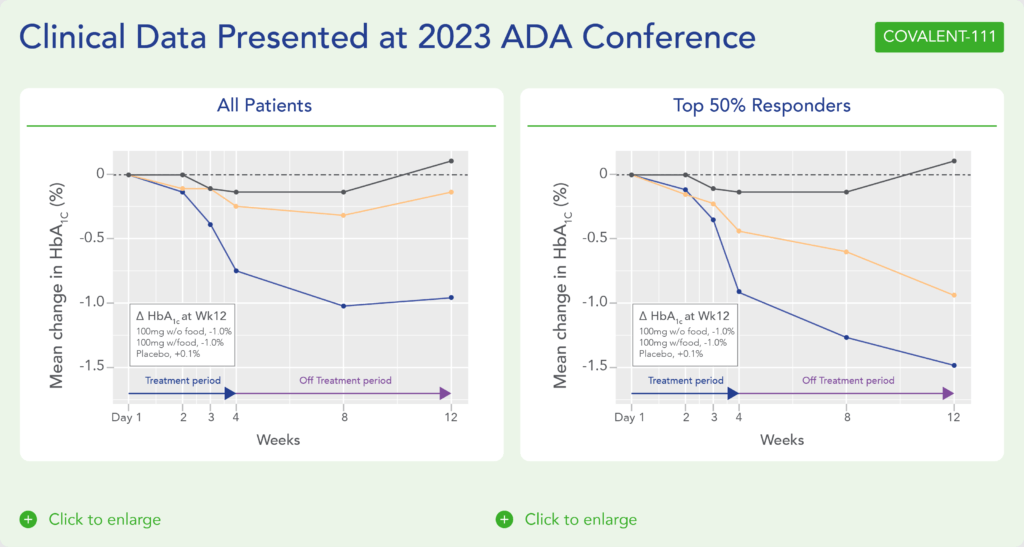
Biomea reported initial clinical data from the first two cohorts of the Phase 2 portion of the T2D trial COVALENT-111 (NCT05731544) in March 2023:
- 89% of patients enrolled in Cohort 3 (n=10 patients at 100 mg dosed daily without food for 4 weeks) achieved a reduction in HbA1c, with 78% achieving ≥ 0.5% reduction and 56% achieving ≥ 1% reduction (median and mean reduction over the entire cohort: -1.0% and -0.81%, respectively).
- Icovamenib was well tolerated and demonstrated a favorable safety profile with no dose discontinuations.
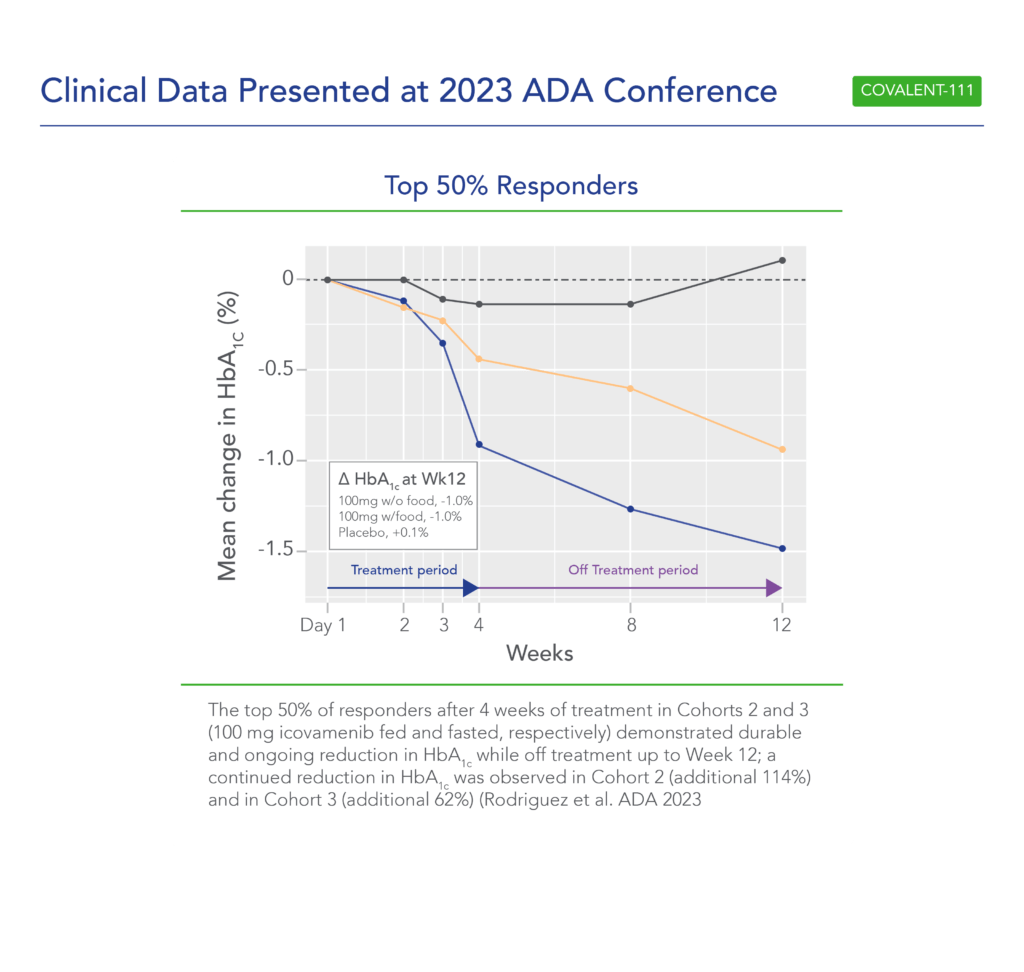
Additional clinical data were presented in June 2023 at the annual meeting of the American Diabetes Association (ADA) from the first two cohorts of patients with T2D enrolled in the Phase 2 portion of COVALENT-111 (Rodriguez et al. ADA 2023):
- This new data highlighted specific patients treated with icovamenib for 4 weeks who maintained or experienced a further decrease in HbA1c levels 8 weeks after treatment was completed, highlighting up to a 2.4% reduction from baseline.

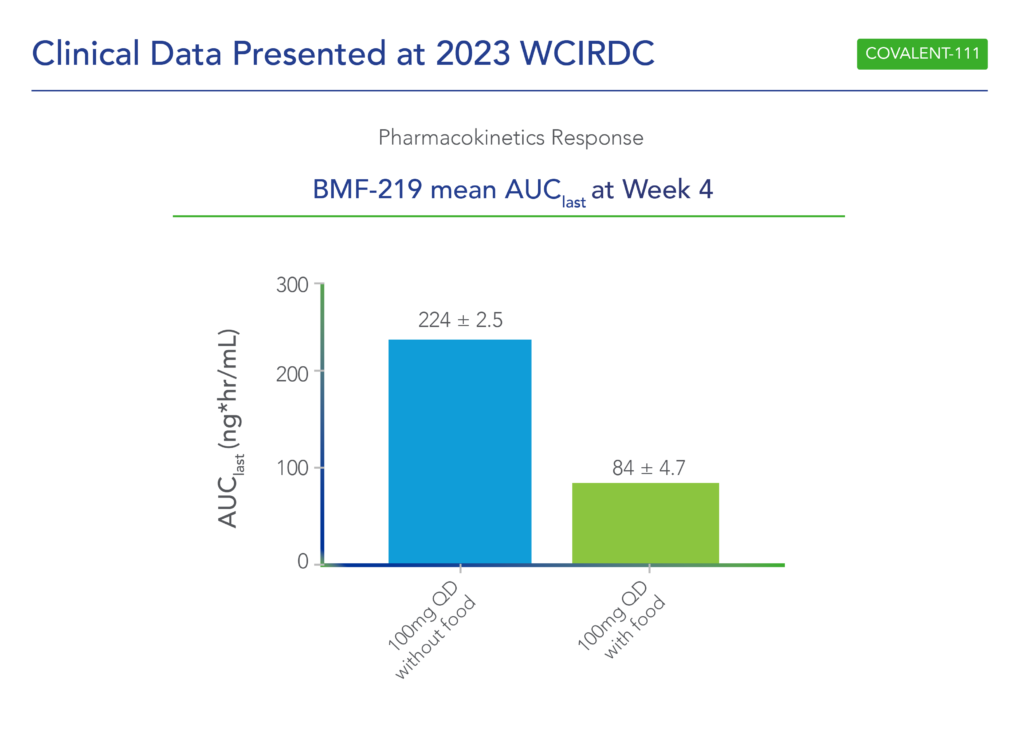
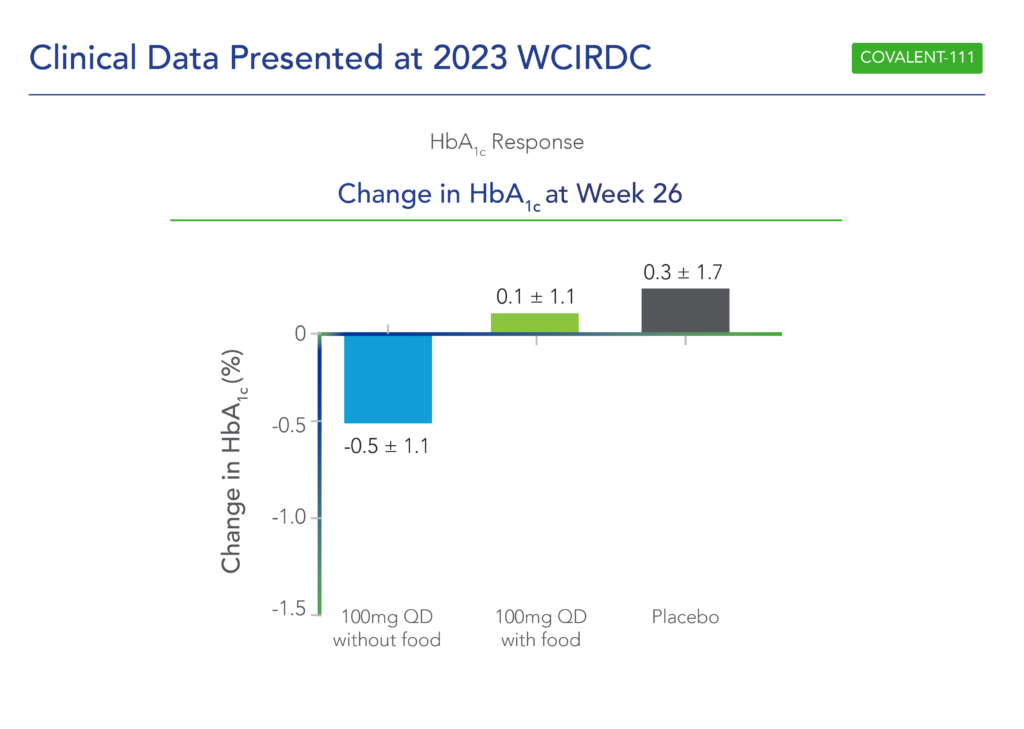
In December 2023 at the World Congress Insulin Resistance Diabetes & Cardiovascular Disease (WCIRDC), Biomea presented long-term follow-up data showing improved glycemic control after 22 weeks off treatment in the ongoing COVALENT-111 study of icovamenib in T2D (Frias et al. WCIRDC 2023):
- At Week 26 – 22 weeks following the last dose of icovamenib – participants in the 100 mg QD (dosed without food) cohort saw a placebo-adjusted mean reduction in HbA1c of 0.8% (compared to a 0.7% placebo-adjusted mean reduction in HbA1c at Week 4, immediately after the completion of icovamenib dosing).
- The observed HbA1C reduction was supported by an increase from baseline in placebo-adjusted mean HOMA-B (+270%) and in mean stimulated C-peptide AUC (+22%) at Week 26 in responders (defined as those T2D subjects achieving HbA1c reduction ≥0.5% at Week 26) and with baseline HOMA-B below the upper limit of normal of <200).
- Icovamenib was generally well tolerated; there were no dose reductions, dose discontinuations, or severe or serious adverse events, and no symptomatic or asymptomatic hypoglycemia was observed.


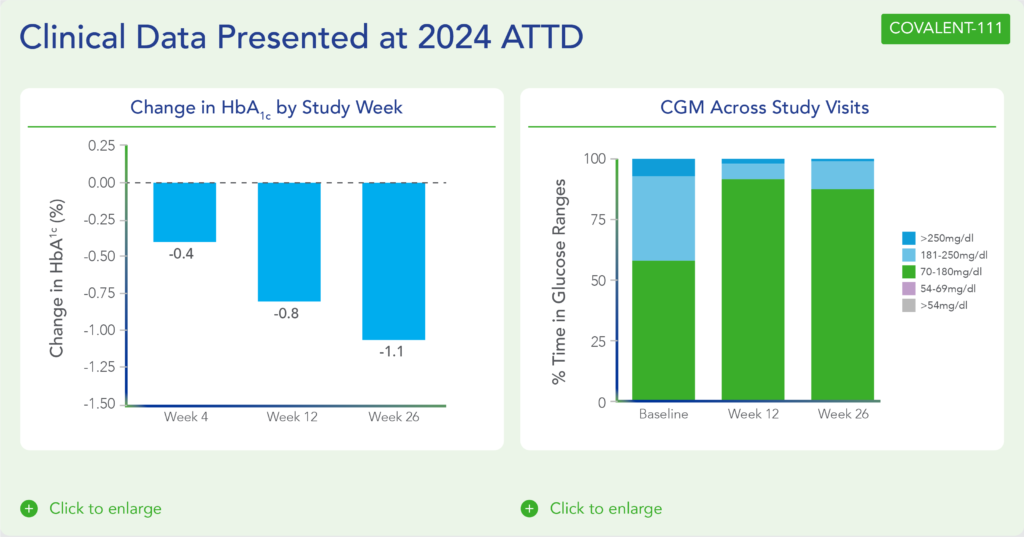
Biomea presented additional clinical data sets from the dose escalation phase of COVALENT-111 in March 2024 at ATTD, highlighting icovamenib’s novel mechanism of action in patients with T2D (Rodriguez et al. ATTD 2024, Abitbol et al. ATTD 2024, Denham et al. ATTD 2024):
- Patients in COVALENT-111 displayed improving glycemic control while off therapy, supporting enhanced pancreatic islet function following icovamenib treatment.
- Consistent with the mechanism of action, patients who demonstrated the greatest HbA1c reduction at Week 26 (22 weeks off treatment) had the greatest improvement in beta cell function as measured by HOMA-B and C-peptide.
- In patients failing current standard of care medications, at Week 26, following only a 4-week dosing cycle of icovamenib, a general dose-response association was observed with placebo-adjusted mean percent changes of HbA1c of -0.04% (50mg QD*), -0.2% (100mg QD with food), -0.8% (100mg without food), -0.4% (200mg QD), -0.4% (100mg BID), and -1.4% (200mg with food) (*50mg data out to Week 20, at the time of data cut).
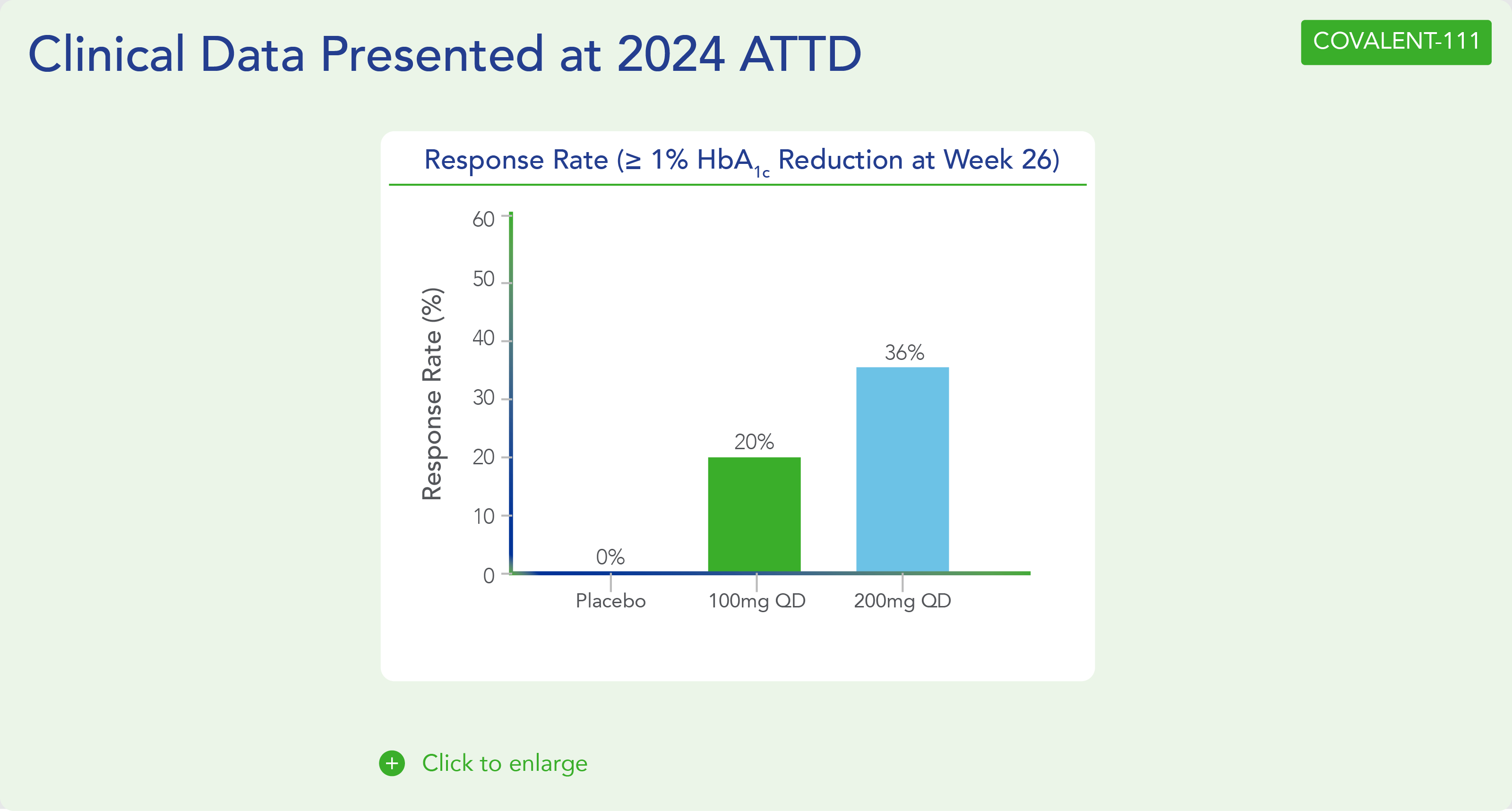
- Across 100mg QD, 200mg QD, and 100mg BID cohorts (N=40), 38% of patients had ≥0.5% HbA1c reduction (with a mean reduction of 1.2%), and 23% of patients had ≥1.0% HbA1c reduction (with a mean reduction of 1.5%) at Week 26.
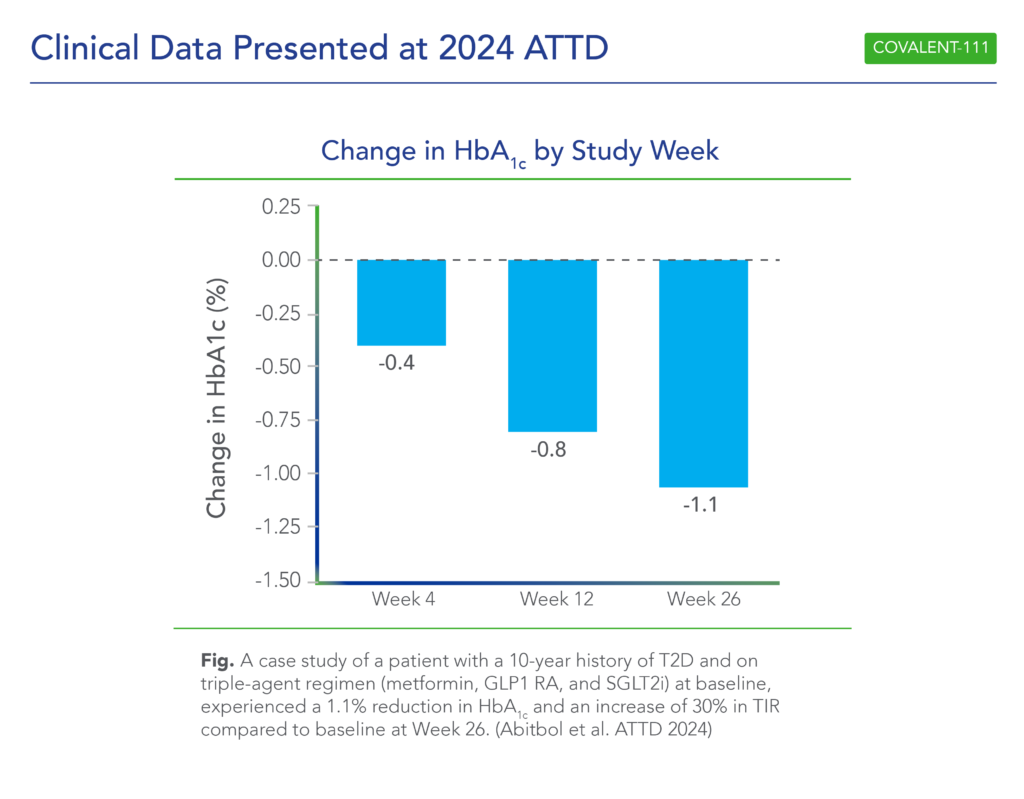
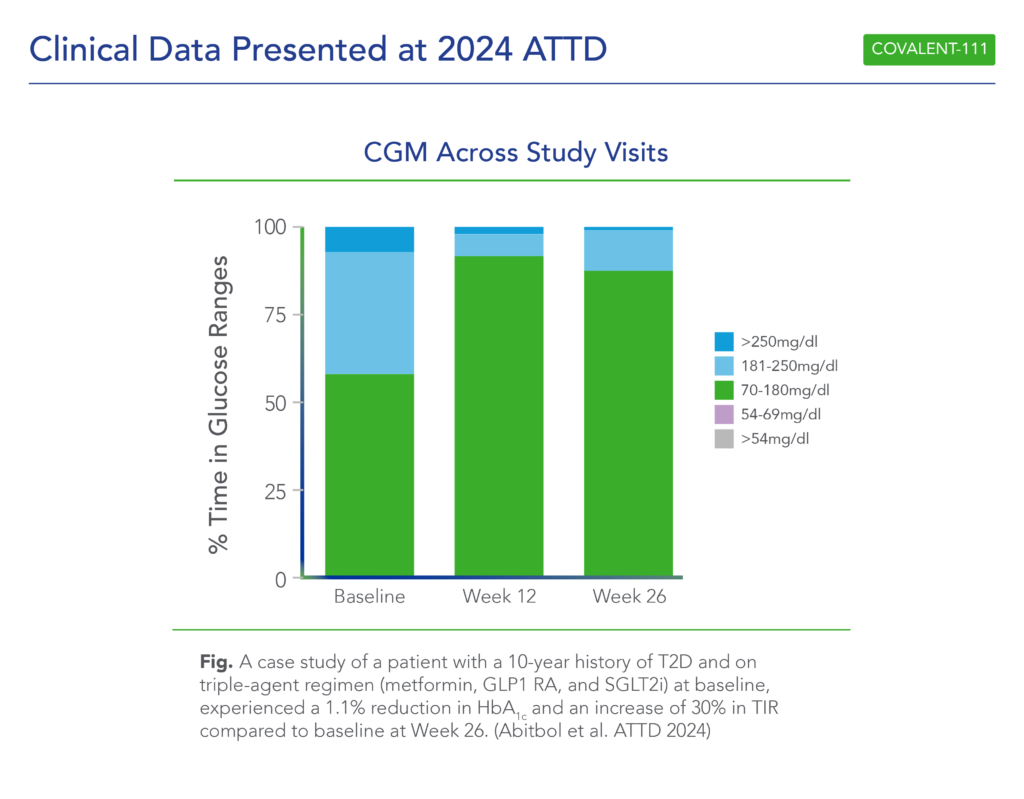
- Patients with >7 years duration of diabetes and failing dual- or triple-agent therapy (including GLP1 RA and/or SGLT2i) (n=2) also demonstrated improved glycemic control (HbA1c -0.4%, -1.1%, and -1.1% at Weeks 4, 12, and 26, respectively) following icovamenib dosing at 200mg with food.
- Increases in HOMA-B and C-peptide generally correlated with glycemic control, consistent with icovamenib’s core mechanism of action: increased beta-cell proliferation and improved beta-cell function.
- Icovamenib was generally well tolerated with no serious adverse events and no adverse event-related study discontinuations, and no symptomatic or clinically significant hypoglycemia.
- 100mg icovamenib dose levels have been selected for the first 3 Arms of the Expansion Phase of COVALENT-111, which will dose patients for up to 12 weeks (compared to 4 weeks in the Escalation Phase) and with extended off-treatment follow-up to Week 52.
- In addition, Biomea reported that the 200 mg icovamenib dosing cohorts nearly doubled the percentage of patients (~36%) achieving durable HbA1c reduction of 1% or more compared to the 100 mg cohorts, reported earlier at 20%.

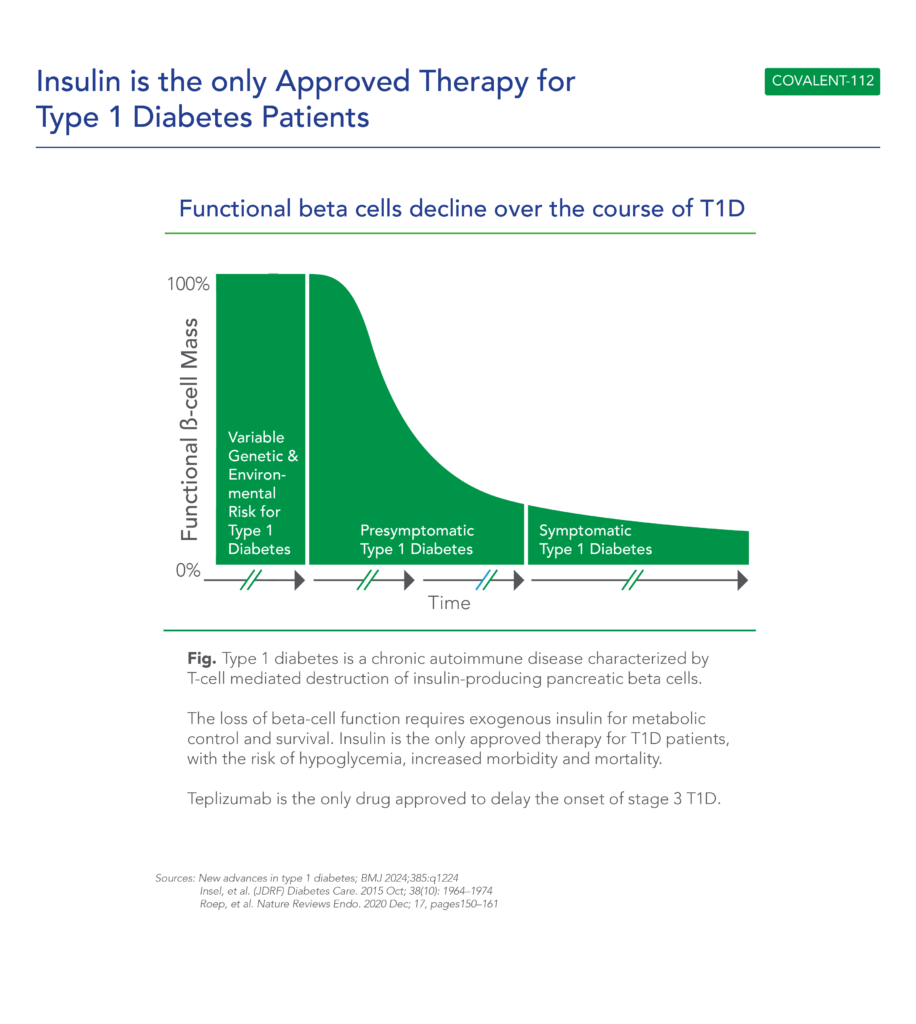
About Type 1 Diabetes
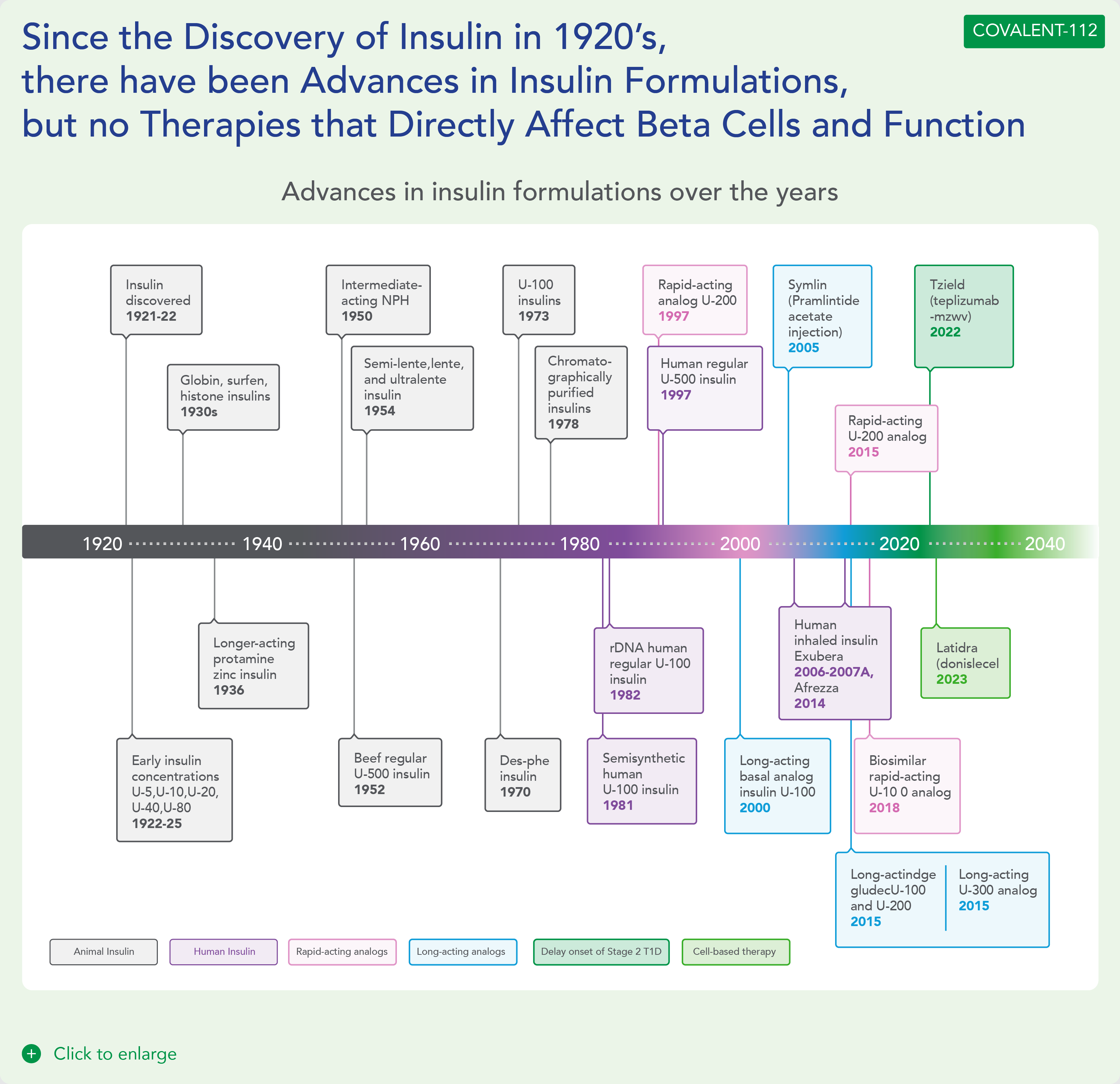
Type 1 Diabetes is a growing disease burden in the US and globally and is associated with significant morbidity. Type 1 diabetes is a chronic autoimmune disease characterized by T-cell mediated destruction of insulin-producing pancreatic beta cells. The loss of beta-cell function requires exogenous insulin for metabolic control and survival. Insulin is the only approved therapy for T1D patients, with the risk of hypoglycemia, increased morbidity and mortality. Since the Discovery of Insulin in 1920’s, there Have Been Advances in Insulin Formulations, but no therapies that directly affect beta cells and their function.

COVALENT-112 (Type 1 Diabetes)
Clinical Design and Results
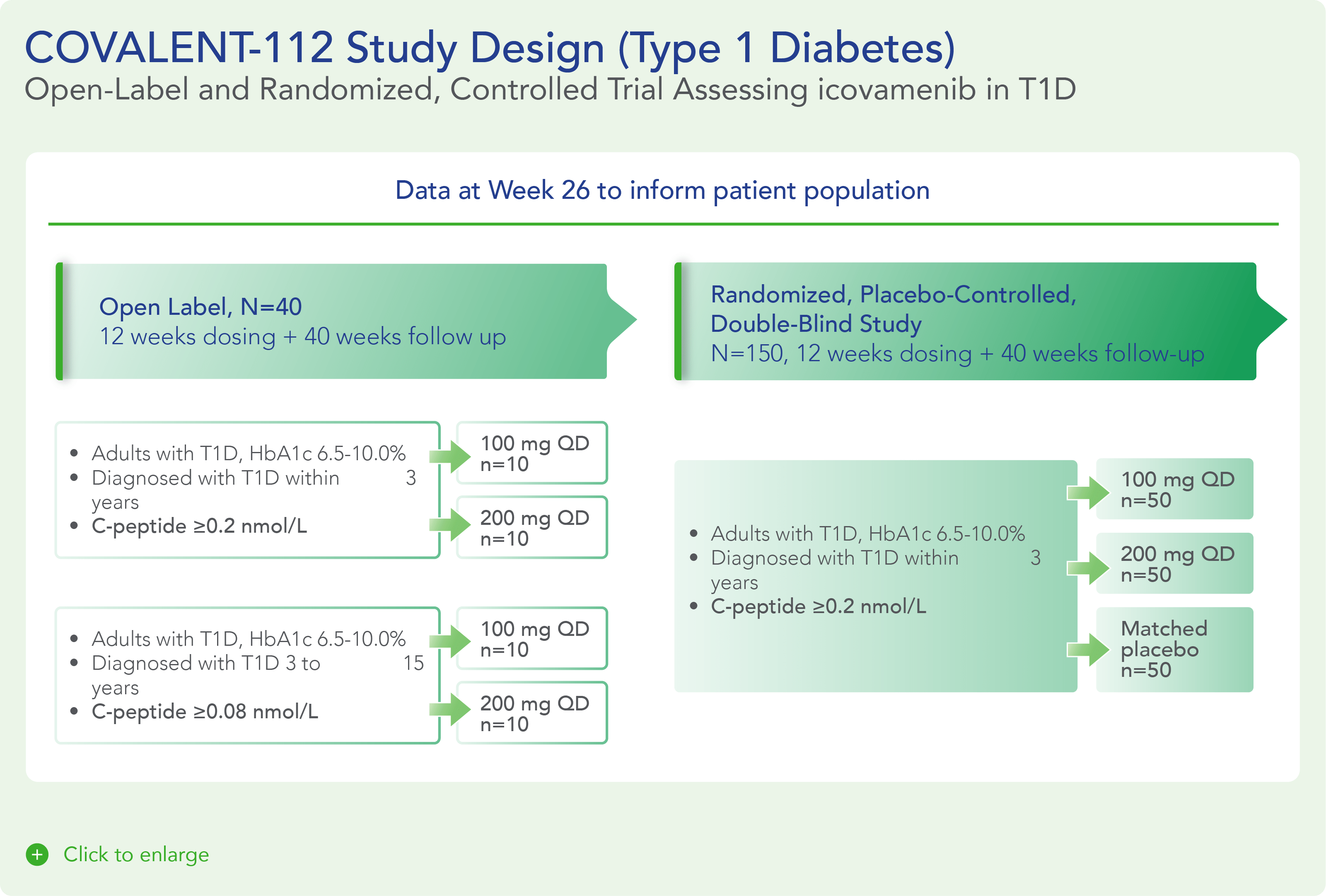
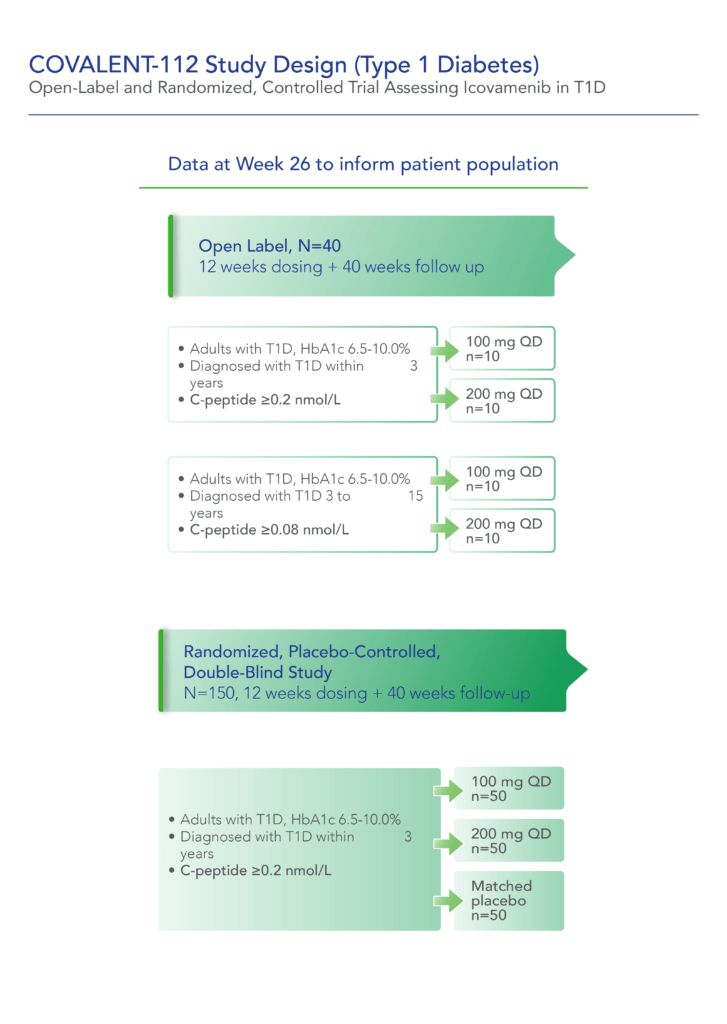
COVALENT-112 is a multi-site, randomized, double-blind, placebo-controlled Phase II study in adults with stage 3 type 1 diabetes. This stage describes the period following clinical diagnosis of type 1 diabetes when symptoms are present due to significant beta cell loss. COVALENT-112 will be enrolling patients in a multi-arm trial comparing two different doses of icovamenib to placebo (1:1:1) to evaluate the safety, tolerability, and efficacy of icovamenib in adults with type 1 diabetes. Approximately 150 patients will be enrolled in the trial and will receive either icovamenib or placebo for 12 weeks, followed by a 40-week off-treatment period. This trial also includes an open-label portion for adults with type 1 diabetes up to 15 years since diagnosis. This Phase II a portion (n=40) will examine the safety, efficacy and durability of icovamenib at two oral dose levels, 100 mg and 200 mg, for 12 weeks of treatment followed by a 40-week off-treatment period.
The first patient in the COVALENT-112 study was dosed at the end of December 2023. In April 2024, Biomea presented data from the first two type 1 diabetes patients enrolled in COVALENT-112, both of whom demonstrated early signs of clinical activity including improved measures of beta-cell function as indicated by c-peptide responses following initial treatment with icovamenib.
Biomea Publications
Literature References
Covalent Inhibition
The article explores the emergence and increasing importance of targeted covalent inhibitors in drug discovery. It discusses how these inhibitors are designed to form durable bonds with specific target proteins, leading to enhanced potency and selectivity compared to traditional non-covalent inhibitors. The review covers key principles in the design and optimization of targeted covalent inhibitors, as well as their applications across various disease areas.
The article addresses the challenges associated with targeting PPIs, which are crucial for cellular signaling and often implicated in cancer progression. It discusses various approaches and techniques employed in the design of covalent PPI inhibitors, emphasizing the need for specificity and potency. Case studies and examples are provided in preclinical and clinical settings, highlighting their potential in overcoming resistance mechanisms and enhancing treatment efficacy in cancer.
This perspective article discusses fundamental concepts such as the mechanisms of covalent binding between drugs and their targets, as well as factors influencing the kinetics of these interactions. The author explores how understanding the kinetic properties of covalent and irreversible inhibitors can inform drug design and optimization processes. The article also addresses the implications of kinetic parameters on efficacy, selectivity, and safety profiles of covalent drugs.
The article discusses the advantages of covalent drugs regarding enhancing potency and selectivity. It highlights the challenges in designing covalent drugs, such as ensuring specificity and minimizing off-target effects to minimize potential toxicity. The review also covers various strategies for optimizing covalent drug candidates, including medicinal chemistry approaches and the use of advanced screening techniques.
Diabetes and Beta Cell Function
The article explores the pivotal role of beta-cell dysfunction and death in the pathophysiology of type 2 diabetes mellitus (T2DM). It discusses how the progressive loss of beta-cell function and mass contributes to the inability to maintain normal blood glucose levels in individuals with T2DM, highlighting factors such as genetic predisposition, lifestyle choices, and environmental factors that influence beta-cell health and function. The review suggests that strategies aimed at preserving beta-cell mass and function could hold promise for preventing and managing T2DM.
The article explores the shared susceptibility and underlying mechanisms of beta-cell dysfunction in both T1DM and T2DM. It discusses how genetic predisposition, autoimmune responses, and environmental factors contribute to the loss of beta-cell function in both forms of diabetes. The review emphasizes future research directions aimed at addressing beta-cell failure as a key strategy in managing and potentially preventing both T1DM and T2DM.
The article explores strategies aimed at achieving remission rather than mere control of T2DM. It discusses various approaches including lifestyle modifications, pharmacotherapy, and bariatric surgery, which have shown potential in achieving sustained remission of T2DM. It also emphasizes personalized treatment plans tailored to individual patient characteristics such as age, duration of diabetes, and comorbidities. The review also covers emerging therapies such as GLP-1 receptor agonists and SGLT-2 inhibitors, which have demonstrated efficacy in improving beta-cell function and insulin sensitivity.
The article discusses how the preservation or restoration of beta-cell mass is crucial for achieving stable blood glucose levels and minimizing complications associated with T1D. It reviewed current research and clinical evidence highlighting that even small residual amounts of beta-cell function can significantly improve glycemic outcomes and reduce the risk of hypoglycemia.
The article discusses how the preservation or restoration of beta-cell mass is crucial for achieving stable blood glucose levels and minimizing complications associated with T1D. It reviewed current research and clinical evidence highlighting that even small residual amounts of beta-cell function can significantly improve glycemic outcomes and reduce the risk of hypoglycemia.
The article examines the utility of the postprandial C-peptide to glucose ratio as an indicator of beta-cell function in individuals with T2D and discusses how this ratio reflects the insulin secretion relative to glucose levels after meals, providing insights into beta-cell health and function beyond fasting measurements. The review suggests that monitoring postprandial C-peptide to glucose ratios could improve the management of T2D by assessing beta-cell responsiveness and predicting treatment outcomes as a valuable tool in personalized diabetes care.
The study explores how reducing fat accumulation in the liver and pancreas is critical for T2D remission. It highlights that while weight loss and fat reduction are crucial, sustained remission also depends on the capacity of beta cells to recover and improve insulin secretion. The findings underscore the complex interplay between metabolic factors and beta-cell health in achieving and maintaining T2D remission, suggesting comprehensive approaches that address both fat accumulation and beta-cell recovery are essential for long-term management of the disease.
The article explores various therapeutic interventions aimed at achieving remission in T2D, including strategies such as lifestyle modifications, pharmacotherapy, and bariatric surgery. It reviewed current research on the mechanisms underlying these interventions, including their effects on insulin sensitivity, beta-cell function, and metabolic pathways. The article emphasizes the importance of personalized treatment approaches tailored to individual patient characteristics and disease progression.
The article continues to explore therapeutic interventions aimed at achieving remission in T2D by focusing on pharmacological agents and their mechanisms of action in improving glycemic control and potentially inducing remission. They discuss the efficacy and safety profiles of various medications, including insulin sensitizers like metformin, GLP-1 receptor agonists, SGLT-2 inhibitors, and other emerging therapies. The review highlights clinical evidence and guidelines for using these agents, emphasizing their roles in reducing cardiovascular risks and enhancing overall metabolic health in T2D patients.
Beta Cell Proliferation
The review explores the phenomenon of pancreatic beta-cell proliferation in the context of obesity. The authors discuss how obesity, characterized by excess adiposity, insulin resistance, and metabolic dysregulation, impacts beta-cell function and mass. They examine mechanisms through which obesity-related factors such as nutrient excess, inflammation, and insulin resistance influence beta-cell proliferation and survival. The review suggests that understanding these mechanisms is crucial for developing strategies to preserve beta-cell function and prevent diabetes in the context of obesity-related metabolic disorders.
The study investigates changes in the structure of the endocrine pancreas during pregnancy. The authors examine pancreatic tissue samples from pregnant women to analyze alterations in the size, distribution, and function of pancreatic islet cells, which are responsible for insulin production. The study underscores the physiological adjustments of the pancreas during gestation, highlighting the relevance for understanding metabolic changes and potential implications for maternal health and fetal development.
The study explores how prolactin, traditionally associated with lactation, influences beta-cell function and proliferation. The authors utilize experimental models to demonstrate that prolactin acts on both beta cells and non-beta cells within the pancreas, enhancing beta-cell mass and insulin secretion capacity. The study highlights that these adaptations are crucial for meeting increased insulin demands during pregnancy, ensuring maternal glucose homeostasis.
The study explores how prolactin signaling activates PBK, a key mediator in promoting the expansion of beta-cell mass in response to the metabolic demands of pregnancy. Using mouse models, they demonstrate that PBK plays a critical role in enhancing beta-cell proliferation, thereby facilitating increased insulin production necessary for maternal glucose regulation during gestation.
The review article discusses how pregnancy induces profound changes in pancreatic beta-cells, leading to their proliferation and expansion. It examines molecular pathways and hormonal factors involved in regulating beta-cell growth, including the roles of prolactin, placental lactogens, and insulin-like growth factors. The review highlights the adaptive responses of beta-cells to the increased metabolic demands during gestation, emphasizing the importance of these mechanisms in maintaining maternal glucose homeostasis.
The article investigates the mechanisms of beta-cell compensation in the context of gestational diabetes mellitus (GDM). The authors explore how pregnancy induces adaptations in beta-cell function to meet increased insulin demands, emphasizing the role of compensatory mechanisms when insulin sensitivity is impaired. The review addresses the implications of these adaptations for the development and management of GDM, highlighting potential targets for therapeutic interventions aimed at preserving beta-cell function and improving maternal and fetal outcomes.
The study examines how serum obtained from pregnant individuals stimulates beta-cell proliferation and enhances insulin secretion in vitro. They utilize experimental models to demonstrate that factors present in pregnancy serum promote the growth and function of beta cells, potentially through signaling pathways involved in cellular proliferation and insulin synthesis.
The article investigates how prolactin, traditionally associated with lactation and reproductive functions, also plays a modulatory role in immune responses and pancreatic beta-cell function in T1D. They discuss experimental evidence suggesting that prolactin may exert protective effects on beta-cells, potentially reducing autoimmune destruction and preserving insulin production. The review proposes implications for therapeutic approaches aimed at modulating prolactin signaling to benefit individuals with T1D.
The study investigates how pregnancy and postpartum periods influence gene expression in different types of pancreatic islet cells. The authors use transcriptomic analysis to examine changes in alpha cells, beta cells, delta cells, and PP cells during these physiological stages. They explore how pregnancy alters the gene expression profiles of these cells, potentially affecting their functions related to hormone secretion and glucose metabolism regulation.
The author discusses the potential protective effect of breastfeeding against the development of diabetes. It highlights epidemiological evidence suggesting that breastfeeding is associated with a lower risk of both T2DM and GDM in women. The review emphasizes that breastfeeding may positively influence maternal glucose metabolism and insulin sensitivity postpartum, contributing to a reduced risk of diabetes later in life.
The study explores the relationship between lactation and the risk of developing T2DM in women with a history of GDM. The authors conduct a systematic review and meta-analysis and found that longer duration of breastfeeding is associated with a reduced incidence of T2DM in this population, suggesting a potential protective effect. The authors discuss mechanisms such as improved glucose metabolism and insulin sensitivity postpartum as contributing factors to this observed association.
The systematic review and meta-analysis synthesized findings from cohort studies to evaluate the impact of breastfeeding duration on T2DM risk post-GDM. Their analysis indicates that longer duration of lactation is associated with a reduced risk of developing T2DM following a pregnancy complicated by GDM. They discuss potential mechanisms such as improved insulin sensitivity and metabolic benefits conferred by breastfeeding.
The prospective cohort study investigates the relationship between lactation duration and the risk of developing T2DM following gestational diabetes mellitus GDM. The authors followed a cohort of women who had a history of GDM and found that longer duration of lactation is associated with a lower risk of developing T2DM. The study highlights that each additional year of breastfeeding was linked to a significant reduction in T2DM risk.
The study investigates the long-term impact of prior lactation on insulin sensitivity and the risk of developing diabetes by conducting a study to examine how past breastfeeding experiences influence metabolic health beyond the lactation period. They found that women with a history of breastfeeding exhibited improved insulin sensitivity compared to those who had not breastfed.
The study synthesized data from observational studies to assess how breastfeeding duration impacts maternal T2DM risk. Their analysis indicates that longer duration of lactation is associated with a lower risk of developing T2DM later in life. The study highlights that each additional year of breastfeeding is linked to a significant reduction in the risk of T2DM, suggesting a dose-response relationship.
The study investigates the long-term impact of lactation duration on the risk of developing diabetes among women over a 30-year period. The authors utilized data from the CARDIA study, a large prospective cohort study, to examine how breastfeeding duration influences the incidence of Type 2 diabetes mellitus T2DM. Their findings reveal that longer cumulative lactation duration is associated with a lower risk of developing T2DM.
The study explores the relationship between breastfeeding duration and the prevalence of impaired fasting glucose (IFG) and diabetes among perimenopausal and postmenopausal women in South Korea. Their findings indicate that longer duration of breastfeeding is associated with a lower prevalence of IFG and diabetes in perimenopausal and postmenopausal women.
Menin and Beta Cell Proliferation
The study demonstrates that menin promotes histone methylation, which in turn enhances the expression of genes encoding p27Kip1 and p18INK4c. These genes are involved in cell cycle regulation and act as tumor suppressors, thereby influencing the growth and proliferation of pancreatic islet cells. The study highlights menin’s role as a critical regulator of pancreatic islet homeostasis through epigenetic mechanisms, providing insights into potential therapeutic targets for diseases involving pancreatic dysfunction.
The study demonstrates that acute deletion of the Men1 gene leads to a significant reversal of preexisting hyperglycemia in these diabetic mice. The Men1 gene is known to regulate pancreatic islet growth and function, and its deletion enhances insulin secretion and improves glucose homeostasis in the experimental animals. This study suggests that targeting Men1 or its downstream pathways could be a potential therapeutic strategy for treating diabetes by restoring pancreatic function and insulin sensitivity.
The study investigates the role of the Men1 gene in preventing hyperglycemia induced by streptozotocin (STZ) in mice. The researchers demonstrate that deletion of the Men1 gene protects mice from developing hyperglycemia when treated with STZ, a compound known to induce pancreatic beta-cell destruction and diabetes. This protective effect is attributed to improved pancreatic islet function and insulin secretion in mice lacking the Men1 gene.
The study demonstrates that high glucose levels suppress the expression of menin in pancreatic beta cells. This repression of menin enhances beta-cell proliferation, suggesting a mechanism by which glucose levels regulate pancreatic islet growth and function. The findings underscore the importance of menin in glucose-mediated signaling pathways that influence beta-cell proliferation and may have implications for understanding and potentially treating conditions involving beta-cell dysfunction, such as diabetes mellitus.
The study demonstrates that menin promotes the growth and proliferation of pancreatic beta-cells in pregnant mice. This enhanced beta-cell growth correlates with increased insulin secretion and glucose intolerance characteristic of GDM. This study provides insights into the molecular mechanisms underlying pancreatic beta-cell adaptation during pregnancy and highlights menin as a potential target for understanding and managing GDM.
The study demonstrates that Menin regulates the expression of Pbk, which in turn promotes beta-cell proliferation in response to HFD-induced metabolic stress. This compensatory proliferation helps maintain beta-cell mass and function under conditions of increased dietary fat intake. The findings suggest that Menin-Pbk signaling pathway is crucial for adapting beta-cell response to dietary challenges, highlighting potential therapeutic targets for metabolic disorders such as obesity and type 2 diabetes mellitus characterized by impaired beta-cell function.
The study focuses on the involvement of Akt, menin, and p21, key regulators known for their roles in cell cycle regulation and growth. The findings highlight that during pregnancy, Akt activation promotes the expression of menin, which in turn modulates the cell cycle inhibitor p21 to facilitate beta-cell proliferation. This orchestrated signaling pathway contributes to the adaptation of pancreatic beta-cells to the increased metabolic demands of pregnancy.
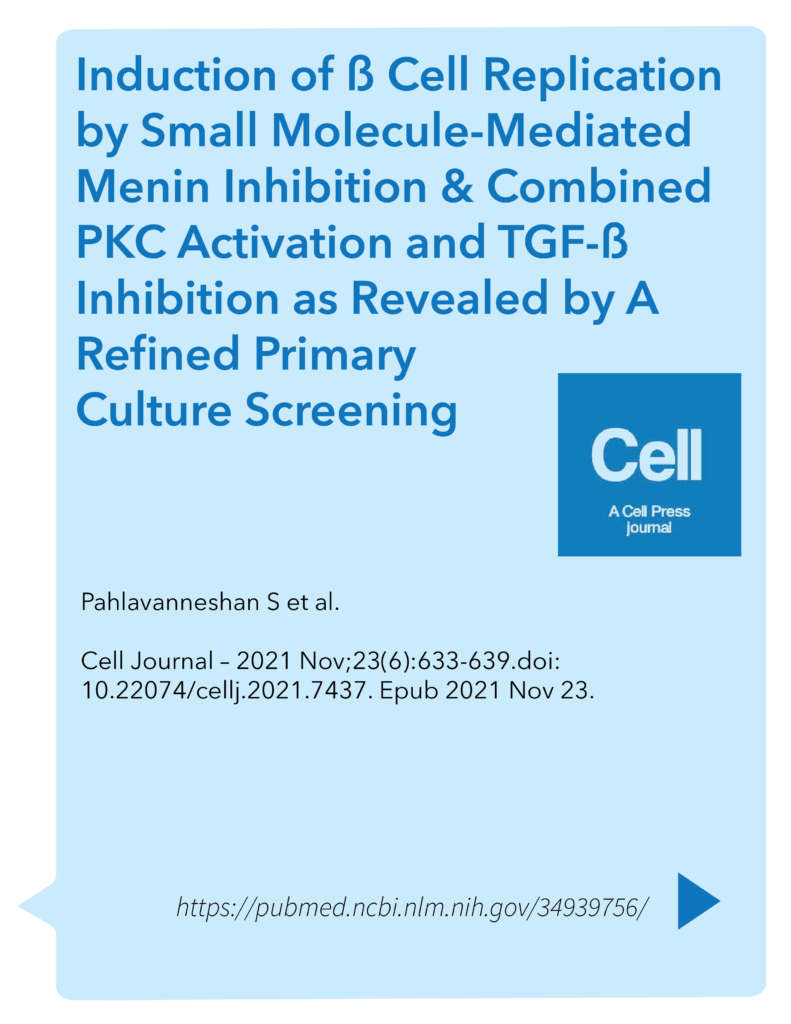
The study focuses on inhibiting the interaction between menin and MLL (mixed-lineage leukemia) proteins, as well as blocking TGF-β signaling, both of which are pathways known to regulate cell proliferation. By combining these inhibitory approaches, the researchers demonstrate an increase in the replication of human pancreatic beta cells in experimental models. This approach suggests a potential therapeutic strategy for promoting beta-cell regeneration in conditions such as diabetes mellitus, where there is a deficit in beta-cell mass and function.
The study focuses on three key interventions: small molecule-mediated inhibition of menin, activation of PKC (protein kinase C), and inhibition of TGF-beta signaling. These pathways are crucial regulators of cell proliferation and differentiation in pancreatic beta cells. The researchers demonstrate that the combined treatment regimen effectively enhances beta cell replication in primary cultures.
The researchers investigate how elevated glucose concentrations impact DNA methylation patterns and histone modifications, which are known to regulate gene expression. Through their research, they identify specific epigenetic alterations associated with prolonged exposure to high glucose, highlighting changes in gene regulatory mechanisms that could contribute to beta cell dysfunction. This study underscores the importance of understanding epigenetic modifications in diabetes pathogenesis and suggests potential targets for therapeutic interventions aimed at preserving or restoring beta cell function in diabetic individuals.
The study sdemonstrate that VGLL4 and MENIN interact with TEAD1, a transcription factor involved in cell proliferation, to suppress its activity. This interaction blocks the expression of genes necessary for beta cell replication, thereby limiting beta cell proliferation. The findings suggest a mechanism by which VGLL4 and MENIN regulate beta cell homeostasis and highlight their potential as targets for therapeutic interventions aimed at modulating beta cell mass in conditions like diabetes mellitus.
The article discusses recent advancements and research efforts aimed at enhancing beta-cell proliferation as a therapeutic strategy for diabetes mellitus. It emphasizes the importance of identifying molecular targets and pathways involved in beta-cell replication, highlighting promising avenues such as modulating signaling pathways and transcription factors.
Type 2 Diabetes Subgroup Analysis
The article presents a study that identifies new subgroups of adult-onset diabetes using a cluster analysis of six key variables, aiming to classify diabetes into more precise categories beyond the traditional type 1 and type 2 distinctions. By analyzing patient data, the researchers discovered distinct subgroups with unique characteristics and varying health outcomes. These findings suggest that tailored treatment approaches could improve management and outcomes for patients with adult-onset diabetes.
The article explores how data-driven subgroups of type 2 diabetes differ in disease progression and treatment response compared to traditional models based on simple clinical features. Using clinical trial data, the researchers identified subgroups with distinct characteristics and treatment responses. The study suggests that these data-driven subgroups offer a more nuanced understanding of type 2 diabetes, potentially leading to more effective and personalized treatment strategies.
The article investigates the classification of type 2 diabetes into distinct subtypes using clinical parameters. By analyzing various clinical data, the researchers identified specific subtypes of type 2 diabetes, each with unique characteristics and health outcomes. This research highlights the potential for a more precise categorization of type 2 diabetes, which could lead to improved patient management and tailored treatment strategies.
The article provides a comprehensive review of studies that use cluster analysis techniques to identify subtypes of diabetes mellitus. The review highlights the various methodologies employed and the different subtypes identified across studies. It emphasizes the potential of cluster analysis to refine diabetes classification, leading to more personalized treatment approaches and better disease management. The systematic review underscores the importance of this technique in enhancing our understanding of diabetes heterogeneity and improving patient outcomes.
The article investigates the underlying causes and distinct characteristics of various subtypes of long-term type 2 diabetes. By examining different patient subgroups, the researchers aim to uncover the specific etiologies contributing to the progression and manifestation of the disease over time. The study highlights the heterogeneity within type 2 diabetes and emphasizes the need for personalized treatment strategies that consider the unique underlying factors of each subtype to improve patient outcomes and disease management.
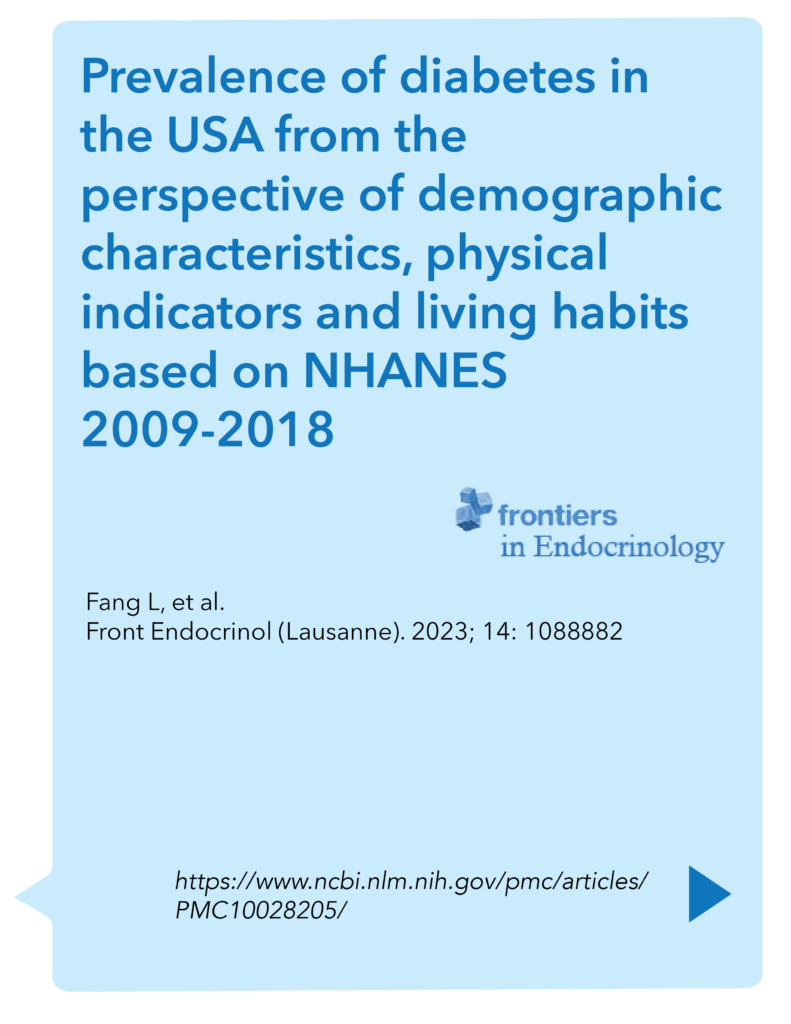
The NHANES data from 2009-2018 reveals that diabetes prevalence in the USA varies significantly across different demographic groups, physical indicators, and living habits. Older adults, particularly those aged 65 and above, have the highest prevalence, and there are notable racial disparities, with non-Hispanic Blacks, Hispanics, and American Indians/Alaska Natives being more affected than non-Hispanic Whites and Asians. Men and women have similar diabetes rates, with a slight predominance in men. Higher BMI and waist circumference are strongly linked to increased diabetes prevalence, highlighting the role of obesity and central fat distribution. Poor dietary habits and low physical activity levels are major contributing factors, while smoking increases diabetes risk and moderate alcohol consumption may offer some protective effects. Addressing these diverse factors is crucial for effective diabetes prevention and management in the US population.
The article discusses innovative approaches to managing blood sugar levels and preventing complications in adult-onset diabetes. By using a clustering-based classification system, the authors propose more personalized treatment strategies tailored to the specific subgroups identified through this method. This perspective emphasizes the potential for improved patient outcomes and more effective prevention of diabetic complications through targeted therapies based on the distinct characteristics of each diabetes subtype.
Menin Science
This study explores how glucagon-like peptide 1 (GLP-1) signaling modulates β cell function by directly influencing the activity of menin, a protein known to suppress insulin expression and β cell proliferation. It demonstrates that protein kinase A (PKA), activated by GLP-1, phosphorylates menin at serine 487, reducing its suppression of insulin production. This phosphorylation event increases menin’s binding affinity to nuclear cytoskeletal proteins, which sequesters it from the Ins1 gene promoter, reducing the recruitment of histone modifiers that typically repress insulin gene expression. As a result, insulin transcription and β cell proliferation increase, revealing a previously unknown physiological link where GLP-1 signaling attenuates menin’s repressive role, potentially offering new insights into diabetes treatment.
This study explores how menin, a regulatory protein, suppresses pancreatic β-cell proliferation by inhibiting GLP1 signaling pathways. Menin and PRMT5, a protein arginine methyltransferase, work together to downregulate the GLP1 receptor (GLP1R) transcription and inhibit protein kinase A (PKA)-mediated phosphorylation of key transcription factors FOXO1 and CREB, which control β-cell mass and gene expression. A menin inhibitor reverses these suppressive actions, promoting GLP1 signaling and β-cell proliferation. Experiments show that menin’s effects extend beyond transcriptional regulation, involving complex molecular interactions that maintain FOXO1 levels and suppress CREB-target genes. Overall, the findings suggest that targeting menin may enhance β-cell mass, potentially benefiting diabetes treatment strategies by increasing β-cell proliferation and improving glucose regulation.
The article discusses the dynamics and functional insights of menin, a protein implicated in various cellular processes. It delves into the molecular interactions and regulatory roles of menin, particularly its partnerships with other proteins and its impact on cellular functions. The study highlights how menin’s interactions influence gene expression and signaling pathways, emphasizing its role as a crucial regulator in cell growth and differentiation.
The study delves into how menin, a protein encoded by the MEN1 gene, regulates various aspects of hematopoietic stem cell differentiation and proliferation. The authors discuss studies that highlight menin’s involvement in maintaining hematopoietic stem cell homeostasis and its impact on lineage commitment towards specific blood cell types. They also examine the consequences of MEN1 gene mutations on hematopoietic function, linking these alterations to diseases like multiple endocrine neoplasia type 1 (MEN1) syndrome.
The study delves into how menin, a protein encoded by the MEN1 gene, regulates various aspects of hematopoietic stem cell differentiation and proliferation. The authors discuss studies that highlight menin’s involvement in maintaining hematopoietic stem cell homeostasis and its impact on lineage commitment towards specific blood cell types. They also examine the consequences of MEN1 gene mutations on hematopoietic function, linking these alterations to diseases like multiple endocrine neoplasia type 1 (MEN1) syndrome.
The study demonstrates that insulin plays a significant role in regulating menin expression, cytoplasmic localization, and its interaction with FOXO1 (Forkhead Box O1), a transcription factor involved in insulin signaling. The study reveals that insulin stimulation leads to increased menin expression and promotes its translocation from the nucleus to the cytoplasm, where it interacts with FOXO1. This interaction is critical in mediating insulin’s effects on gene expression and cellular responses related to glucose homeostasis and insulin sensitivity.
In the review, Menin is highlighted as a key regulator influencing various cellular processes essential for liver and bile duct function, including transcriptional regulation, cell cycle control, and DNA repair mechanisms. The review emphasizes menin’s interaction with numerous transcription factors and chromatin-modifying enzymes, underscoring its ability to modulate gene expression critical for hepatobiliary homeostasis. The authors discuss the implications of MEN1 gene mutations, which are implicated in the pathogenesis of primary liver cancers and bile duct disorders. They explore menin’s involvement in signaling pathways crucial for liver development and its responses to pathological stimuli.
The review examines the role of the menin pathway in epigenetic regulation. Menin, encoded by the MEN1 gene, serves as a scaffold protein that interacts with histone modifiers to regulate chromatin structure and gene expression. Dysregulation of the menin pathway, often due to MEN1 mutations, disrupts these epigenetic mechanisms, contributing to tumorigenesis and cancer progression.




































![Literature References Covalent Inhibition Diabetes and Beta Cell Function Beta Cell Proliferation Menin and Beta Cell Proliferation Type 2 Diabetes Subgroup Analysis Menin in Hematological Malignancies Biomea Publications: Diabetes Biomea Publications: Oncology Covalent Inhibition The article discusses recent innovations in covalent drug discovery, covering various aspects such as the design principles, mechanisms of action, and applications of covalent drugs. It emphasized how advancements in chemical biology and medicinal chemistry have enabled the development of covalent drugs that target specific disease mechanisms with enhanced potency and selectivity. The review also highlights the potential of covalent drugs in treating challenging diseases in the future. The article explores the emergence and increasing importance of targeted covalent inhibitors in drug discovery. It discusses how these inhibitors are designed to form durable bonds with specific target proteins, leading to enhanced potency and selectivity compared to traditional non-covalent inhibitors. The review covers key principles in the design and optimization of targeted covalent inhibitors, as well as their applications across various disease areas. The article addresses the challenges associated with targeting PPIs, which are crucial for cellular signaling and often implicated in cancer progression. It discusses various approaches and techniques employed in the design of covalent PPI inhibitors, emphasizing the need for specificity and potency. Case studies and examples are provided in preclinical and clinical settings, highlighting their potential in overcoming resistance mechanisms and enhancing treatment efficacy in cancer. This perspective article discusses fundamental concepts such as the mechanisms of covalent binding between drugs and their targets, as well as factors influencing the kinetics of these interactions. The author explores how understanding the kinetic properties of covalent and irreversible inhibitors can inform drug design and optimization processes. The article also addresses the implications of kinetic parameters on efficacy, selectivity, and safety profiles of covalent drugs. The article discusses the advantages of covalent drugs regarding enhancing potency and selectivity. It highlights the challenges in designing covalent drugs, such as ensuring specificity and minimizing off-target effects to minimize potential toxicity. The review also covers various strategies for optimizing covalent drug candidates, including medicinal chemistry approaches and the use of advanced screening techniques. [back to top] Diabetes and Beta Cell Function Type 2 diabetes-a matter of beta-cell life and death? Christopher J. Rhodes Science – 2005 Jan 21, 307(5708):380-4. doi: 10.1126/science.1104345. PMID: 15662003. https://pubmed.ncbi.nlm.nih.gov/15662003/ The article explores the pivotal role of beta-cell dysfunction and death in the pathophysiology of type 2 diabetes mellitus (T2DM). It discusses how the progressive loss of beta-cell function and mass contributes to the inability to maintain normal blood glucose levels in individuals with T2DM, highlighting factors such as genetic predisposition, lifestyle choices, and environmental factors that influence beta-cell health and function. The review suggests that strategies aimed at preserving beta-cell mass and function could hold promise for preventing and managing T2DM. Diabetes Invest_Beta-cell failure in diabetes – Common susceptibility and mechanisms shared between type 1 and type 2 diabetes 1 Hiroshi Ikegami, Naru Babaya, and Shinsuke Noso Journal of Diabetes Investigation – 2021 Sep; 12(9): 1526–1539. Published online 2021 Jun 16. doi: 10.1111/jdi.13576 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8409822/ The article explores the shared susceptibility and underlying mechanisms of beta-cell dysfunction in both T1DM and T2DM. It discusses how genetic predisposition, autoimmune responses, and environmental factors contribute to the loss of beta-cell function in both forms of diabetes. The review emphasizes future research directions aimed at addressing beta-cell failure as a key strategy in managing and potentially preventing both T1DM and T2DM. Not control but conquest – Strategies for the remission of Type 2 diabetes mellitus Jinyoung Kim, Hyuk-Sang Kwon Diabetes and Metabolism Journal – 2022 Mar;46(2):165-180. doi: 10.4093/dmj.2021.0377. https://pubmed.ncbi.nlm.nih.gov/35385632/ The article explores strategies aimed at achieving remission rather than mere control of T2DM. It discusses various approaches including lifestyle modifications, pharmacotherapy, and bariatric surgery, which have shown potential in achieving sustained remission of T2DM. It also emphasizes personalized treatment plans tailored to individual patient characteristics such as age, duration of diabetes, and comorbidities. The review also covers emerging therapies such as GLP-1 receptor agonists and SGLT-2 inhibitors, which have demonstrated efficacy in improving beta-cell function and insulin sensitivity. Increased beta-cell proliferation before immune cell invasion prevents progression of Type 1 diabetes Dirice E et al., 2019 Nature Metabolism Nat Metab – 2019 May; 1(5): 509–518. Published online 2019 May 6. doi: 10.1038/s42255-019-0061-8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6696912/ The study explores how enhanced beta-cell replication prior to immune cell infiltration can mitigate the development of T1D. The researchers utilize mouse models and human pancreatic tissue to demonstrate that beta-cells exhibit increased proliferation in response to specific conditions, which leads to improved glucose tolerance and delays in T1D onset. The study underscores the potential therapeutic implications of promoting beta-cell proliferation as a strategy to prevent or delay the progression of T1D. Importance of beta cell mass for glycaemic control in people with Type 1 diabetes Theodorus J P Jansen, et. al., Diabetologia, 2023 Feb; 66(2):367-375. doi: 10.1007/s00125-022-05830-2. Epub 2022 Nov 17. https://pubmed.ncbi.nlm.nih.gov/36394644/ The article discusses how the preservation or restoration of beta-cell mass is crucial for achieving stable blood glucose levels and minimizing complications associated with T1D. It reviewed current research and clinical evidence highlighting that even small residual amounts of beta-cell function can significantly improve glycemic outcomes and reduce the risk of hypoglycemia. Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes Yoshifumi Saisho International Journal of Molecular Science – 2016 May 17; 17(5):744. doi: 10.3390/ijms17050744. PMID: 27196896; PMCID: PMC4881566. https://pubmed.ncbi.nlm.nih.gov/27196896/ The article examines the utility of the postprandial C-peptide to glucose ratio as an indicator of beta-cell function in individuals with T2D and discusses how this ratio reflects the insulin secretion relative to glucose levels after meals, providing insights into beta-cell health and function beyond fasting measurements. The review suggests that monitoring postprandial C-peptide to glucose ratios could improve the management of T2D by assessing beta-cell responsiveness and predicting treatment outcomes as a valuable tool in personalized diabetes care. Remission of human Type 2 diabetes requires decrease on liver and pancreas fat content, but is dependent upon capacity for beta cell recovery Taylor R et al. Cell Metabolism, 2018 Oct 2; 28(4):547-556.e3. doi: 10.1016/j.cmet.2018.07.003. https://pubmed.ncbi.nlm.nih.gov/30078554/ The study explores how reducing fat accumulation in the liver and pancreas is critical for T2D remission. It highlights that while weight loss and fat reduction are crucial, sustained remission also depends on the capacity of beta cells to recover and improve insulin secretion. The findings underscore the complex interplay between metabolic factors and beta-cell health in achieving and maintaining T2D remission, suggesting comprehensive approaches that address both fat accumulation and beta-cell recovery are essential for long-term management of the disease. Intervention with therapeutic agents, Understanding the path to remission in Type 2 Diabetes: Part 1](https://biomeafusion.com/wp-content/uploads/2024/08/Diabetes-and-Beta-Cell-Function_BC_Blue-08-791x1024.png)