Diabetes
Diabetes Pipeline
The following table summarizes our product candidate pipeline. We own full worldwide development and commercialization rights to all of our programs.

All our assets are in house designed, developed, and wholly owned by Biomea Fusion Inc.
BMF219 - Menin Inhibitor in Diabetes
BMF-219, is an orally bioavailable, potent and selective covalent inhibitor of menin, built from our FUSION™ System. Loss of functional beta cell mass is a core component of the natural history in both types of diabetes — type 1 diabetes (mediated by autoimmune dysfunction) and type 2 diabetes (mediated by metabolic dysfunction). Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta cell mass and function have been observed to be diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on beta-cell turnover and growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells.
BMF-219’s proposed mechanism of action in diabetes is to enable the proliferation, preservation, and reactivation of a patient’s own healthy, functional, insulin-producing beta cells. As the potentially first disease-modifying therapy for type 1 and type 2 diabetes, BMF-219 could be an important addition and complement to the treatment landscape, if approved.
Clinical Development
In March 2023, Biomea reported initial clinical data from the first two cohorts of the Phase II portion of COVALENT-111 (NCT05731544). As reported, 89% of patients enrolled in Cohort 3 (n=10 patients at 100 mg without food) achieved a reduction in HbA1c, 78% achieved ≥ 0.5% reduction in HbA1c and 56% achieved ≥ 1% reduction in HbA1c (median and mean reduction over the cohort: -1.0% and -0.81%, respectively). BMF-219 was well tolerated and demonstrated a favorable safety profile with no dose discontinuations.
In June 2023, additional clinical data was presented from the first two cohorts of patients with T2D enrolled in the Phase II portion of COVALENT-111. New data highlighted specific patients treated with BMF-219 for 4 weeks maintained or experienced a further decrease in HbA1c levels 8 weeks after treatment was completed, up to a 2.4% reduction from baseline. (Rodriguez et al. ADA 2023)
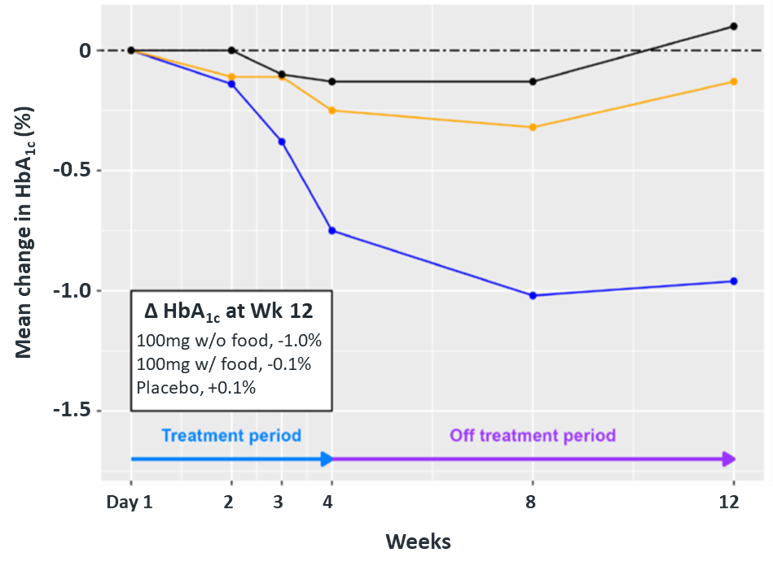
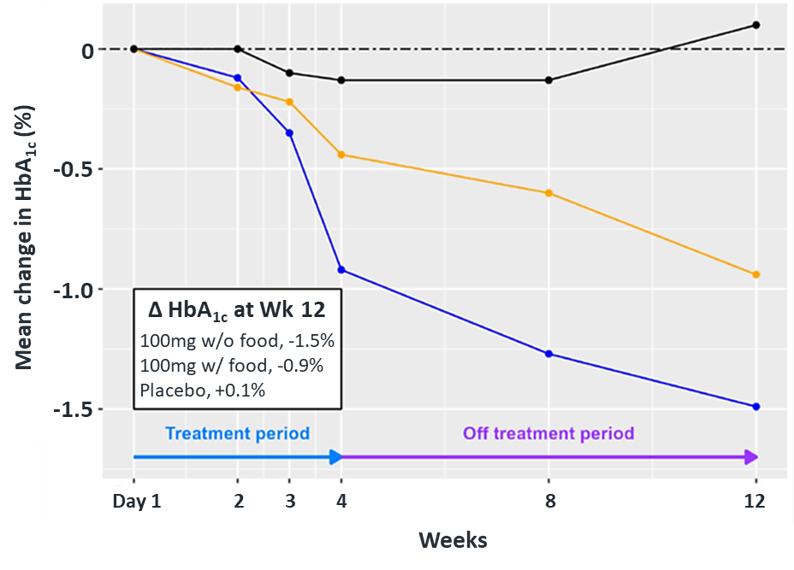
Fig. Clinical data presented at 2023 ADA. The top 50% of responders after 4-weeks of treatment in Cohorts 2 and 3 (100 mg BMF-219 fed and fasted, respectively) demonstrated durable and ongoing reduction in HbA1c while off treatment up to Week 12; a continued reduction in HbA1c was observed in Cohort 2 (additional 114%) and in Cohort 3 (additional 62%) (Rodriguez et al. ADA 2023)
In December 2023, Biomea presented long-term follow-up data showing improved glycemic control after 22 weeks off treatment in ongoing Phase 2 study (COVALENT-111) of BMF-219 in type 2 diabetes. At Week 26, 22 weeks after the last dose of BMF-219, participants in the 100 mg QD (without food) cohort saw an improved placebo adjusted mean reduction in HbA1c of 0.8% (compared to a 0.7% placebo adjusted mean reduction in HbA1c at Week 4). Observed HbA1C reduction was supported by an increase from baseline in placebo adjusted mean HOMA-B (+270%) and in mean stimulated C-peptide AUC (+22%) at Week 26 in responders (defined as HbA1c reduction ≥0.5% at Week 26) with baseline below the HOMA-B upper limit of normal (<200). BMF-219 was generally well tolerated; no dose reductions, dose discontinuations, or severe or serious adverse events and no symptomatic or asymptomatic hypoglycemia was observed. In addition, Biomea reported that the 200 mg cohorts near doubled the percentage of patients (~36%) with durable HbA1c reduction of 1% or more compared to the 100 mg cohorts which reported earlier as 20%. (Frias et al. WCIRDC 2023)
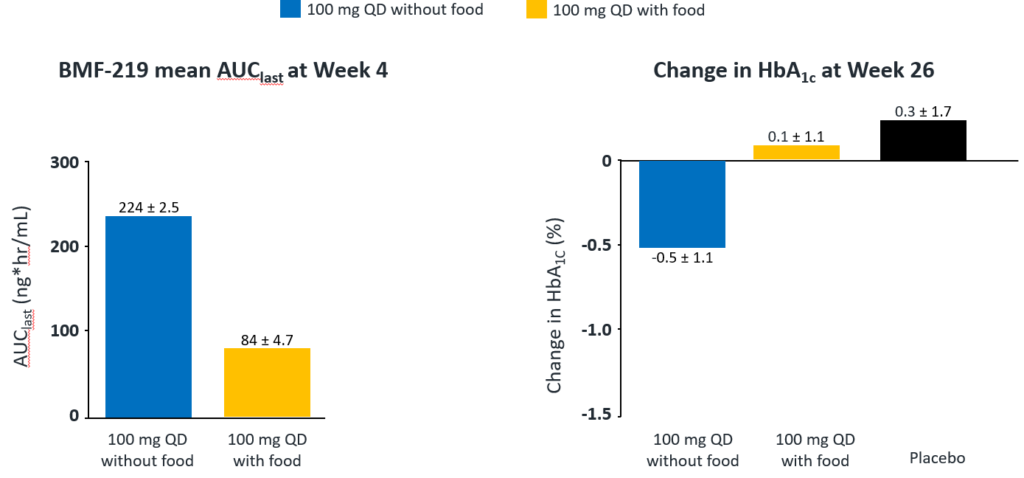
Fig. Clinical data presented at 2023 WCIRDC. Pharmacokinetics and HbA1c Response
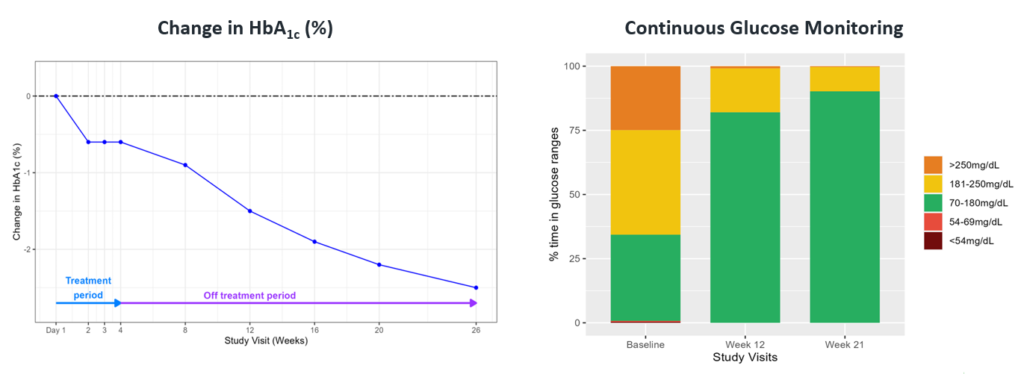
Fig. A case study of a 29-year-old man with 4-year history of T2D
Preclinical Development
Biomea conducted two diabetes animal experiments to measure the potential impact of BMF-219 for the treatment of type 2 diabetes; the Zucker Diabetic Fatty (ZFD) Rat, a widely studied model of obesity and insulin resistance in rats, and the Streptozotocin (STZ) induced rat, a widely studied model by which diabetes is induced using an antibiotic (STZ) that produces pancreatic islet β-cell destruction. In both models, BMF-219 was able to normalize glucose levels in the majority of animals after just two weeks of treatment. Notably, the majority of the effect was maintained despite complete washout of BMF-219. (Butler et al, EASD 2022; Somanath et al. EASD 2022).
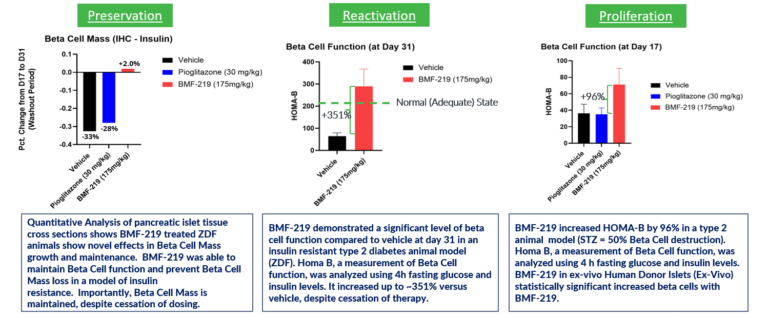
Fig. BMF-219 Regenerated, Preserved and Reactivated Beta Cells in Preclinical Studies
In December 2023, preclinical ex-vivo human islet data was presented. Dependent on dose concentration and also dependent on dose duration, BMF-219 was observed to increase beta cell mass and function, as well as promote controlled proliferation and enhance insulin content in beta cells. Proliferation was observed only under elevated glucose conditions, which mimics diabetic levels, and with continuous drug exposure. BMF-219 was observed to upregulate the expression of key cell-cycle proteins, PbK and CCNA2 (Cyclin A2) in a glucose-dependent fashion. When not sequestered to menin, PbK expression was known to be upregulated by JunD, which is a glucose-sensitive menin binding partner.
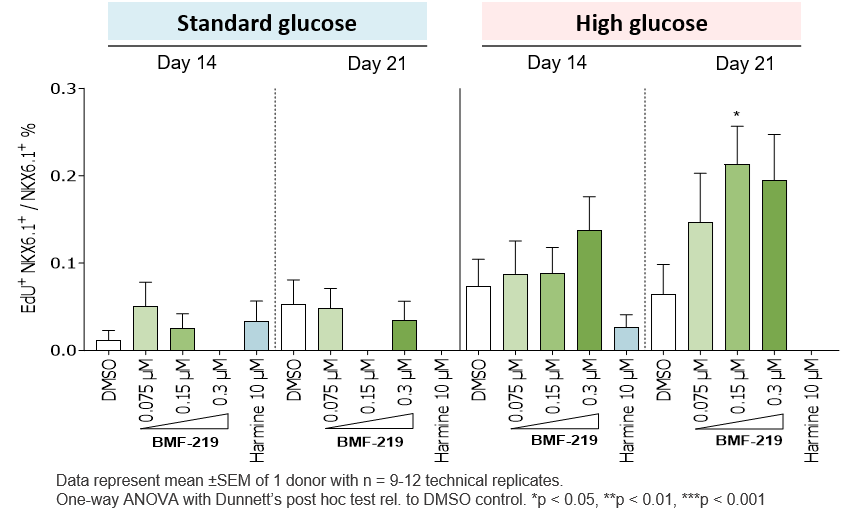
Fig. BMF-219 Induced a Glucose-Dependent Enhancement in β-Cell Proliferation
ADA TV Biomea Film
Learn more about COVALENT-111, a phase 1 / 2 clinical trial in healthy adults and adults with type 2 diabetes.
ADA 2022: BMF219 Preclinical Development Progress
The American Diabetes Association (ADA) featured Biomea during its yearly
“Thought Leadership Film Series” of innovative therapies at its 82nd Annual Meeting.
