Diabetes
CONTENTS
SIDEBAR-CONTENT-1
Pipeline
The following table summarizes our product candidate pipeline. We own full worldwide development and commercialization rights to all of our programs.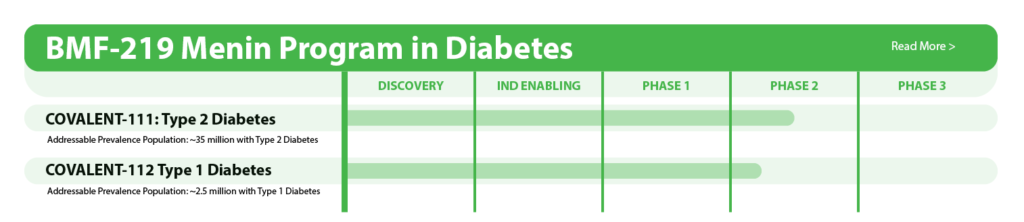
All our assets are in house designed, developed, and wholly owned by Biomea Fusion Inc.
About Diabetes
Diabetes is a chronic health condition that affects the how the body metabolizes nutrients. It results in too much glucose (sugar) in the bloodstream. Over time, this can cause serious health problems by damaging vital tissues and organs. Most people with diabetes have a shorter life expectancy than people without this disease. The Centers for Disease Control (CDC) estimates about 2 in 5 adults in the USA are expected to develop diabetes during their lifetime. More than 37 million people of all ages (about 11% of the US population) have diabetes today. 96 million adults (more than 1 in 3) have prediabetes, glucose levels that are higher than normal but not high enough to be classified as diabetes. Diabetes is also one of the largest economic burdens on the US health care system with $1 out of every $4 in US health care costs being spent on caring for people with diabetes.
Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta-cell mass and function are diminished, leading to insufficient insulin secretion and elevated glucose levels (hyperglycemia).
There are two major types of diabetes: type 1 diabetes (T1D) and type 2 diabetes (T2D). T1D is caused by an autoimmune reaction (the body “attacks itself” by mistake). This reaction destroys insulin producing beta cells in the pancreas, stopping the body’s ability to produce and secrete insulin. Approximately 5-10% of the people who have diabetes have T1D. With T2D, the body doesn’t use insulin well and can’t keep blood sugar at normal levels. About 90-95% of people with diabetes have T2D. Loss of functional beta-cell mass is a core component of the natural history in both T1D and T2D.
Despite significant advances in pharmacotherapy and diabetes-related devices over the past 2 decades, it is estimated that approximately 50% of persons with diabetes in the US do not have adequate glucose control, as defined by a glycated hemoglobin (HbA1C) of 7% or less. One important reason is that current agents for the management of T2D and T1D do not address the root cause of diabetes – a progressive decline in beta-cell mass and function.
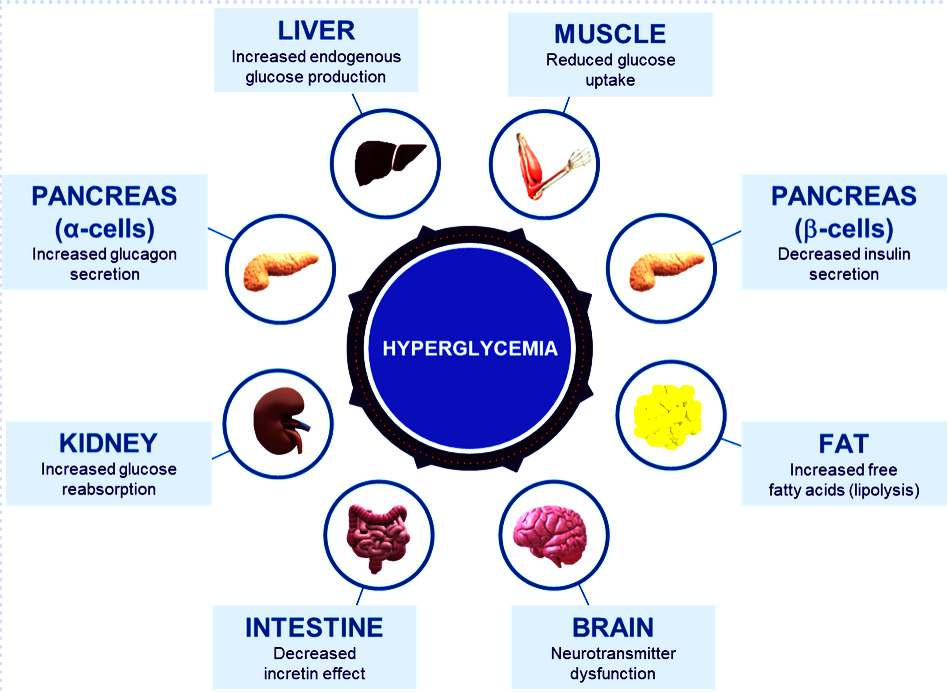
Fig. Different abnormalities that contribute to the hyperglycemia that occurs in patients with T2D (adapted from DeFronzo et al. Diabetes 2009)
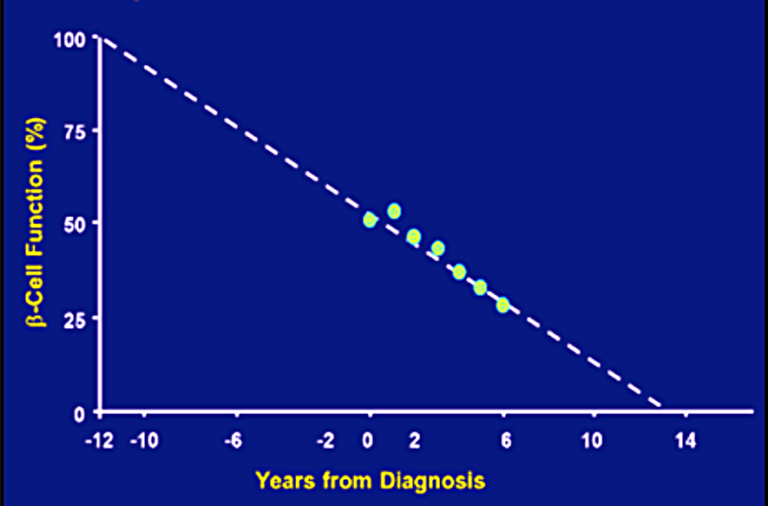 Fig. Progressive decline in beta-cell function (Lebovitz et al. Diabetes Review 1999)
Fig. Progressive decline in beta-cell function (Lebovitz et al. Diabetes Review 1999)
SIDEBAR-CONTENT-2
Menin in Diabetes
Menin is a transcriptional scaffold protein that controls the expression and activity of proteins that regulate beta-cell proliferation. Menin is thought to control beta-cell proliferation and mass by acting as a brake on beta-cell turnover / beta-cell growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells, which could be a disease-modifying approach to treat diabetes.
BMF-219 – Mechanism of Action
BMF-219, is an orally bioavailable, potent and selective covalent inhibitor of menin, built from our FUSION™ System. Loss of functional beta cell mass is a core component of the natural history in both types of diabetes — type 1 diabetes (mediated by autoimmune dysfunction) and type 2 diabetes (mediated by metabolic dysfunction). Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps control blood glucose levels. In patients with diabetes, beta cell mass and function have been observed to be diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on beta-cell turnover and growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells.
BMF-219’s proposed mechanism of action in diabetes is to enable the proliferation, preservation, and reactivation of a patient’s own healthy, functional, insulin-producing beta cells. As the potentially first disease-modifying therapy for type 1 and type 2 diabetes, BMF-219 could be an important addition and complement to the treatment landscape, if approved.
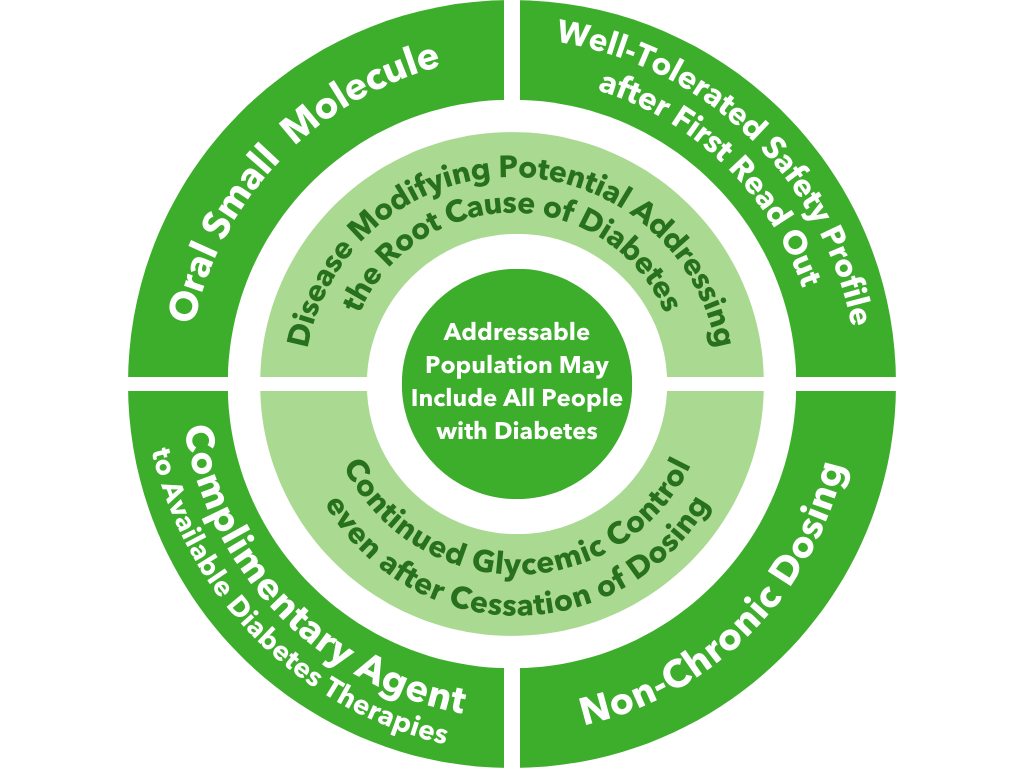
Fig. BMF-219’s unique value proposition
Preclinical Development
Biomea conducted two diabetes animal experiments to measure the potential impact of BMF-219 for the treatment of type 2 diabetes: the Zucker Diabetic Fatty (ZFD) Rat, a widely studied model of obesity and insulin resistance in rats, and the Streptozotocin (STZ) induced rat, a widely studied model by which diabetes is induced using an antibiotic (STZ) that destroys beta cells. In both models, BMF-219 was able to normalize glucose levels in the majority of animals after just 2 weeks of treatment. Notably, the majority of the effect was maintained despite complete washout of BMF-219. (Butler et al, EASD 2022; Somanath et al. EASD 2022).
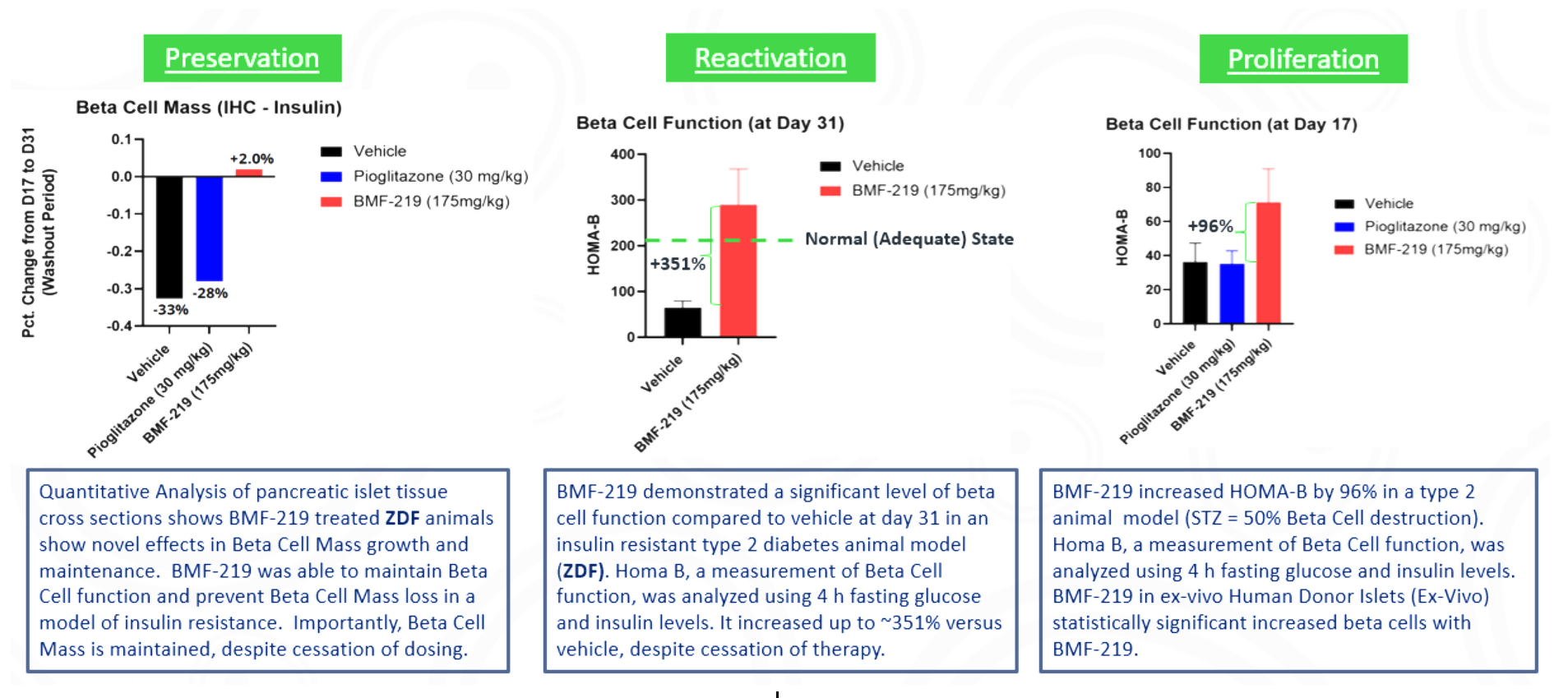
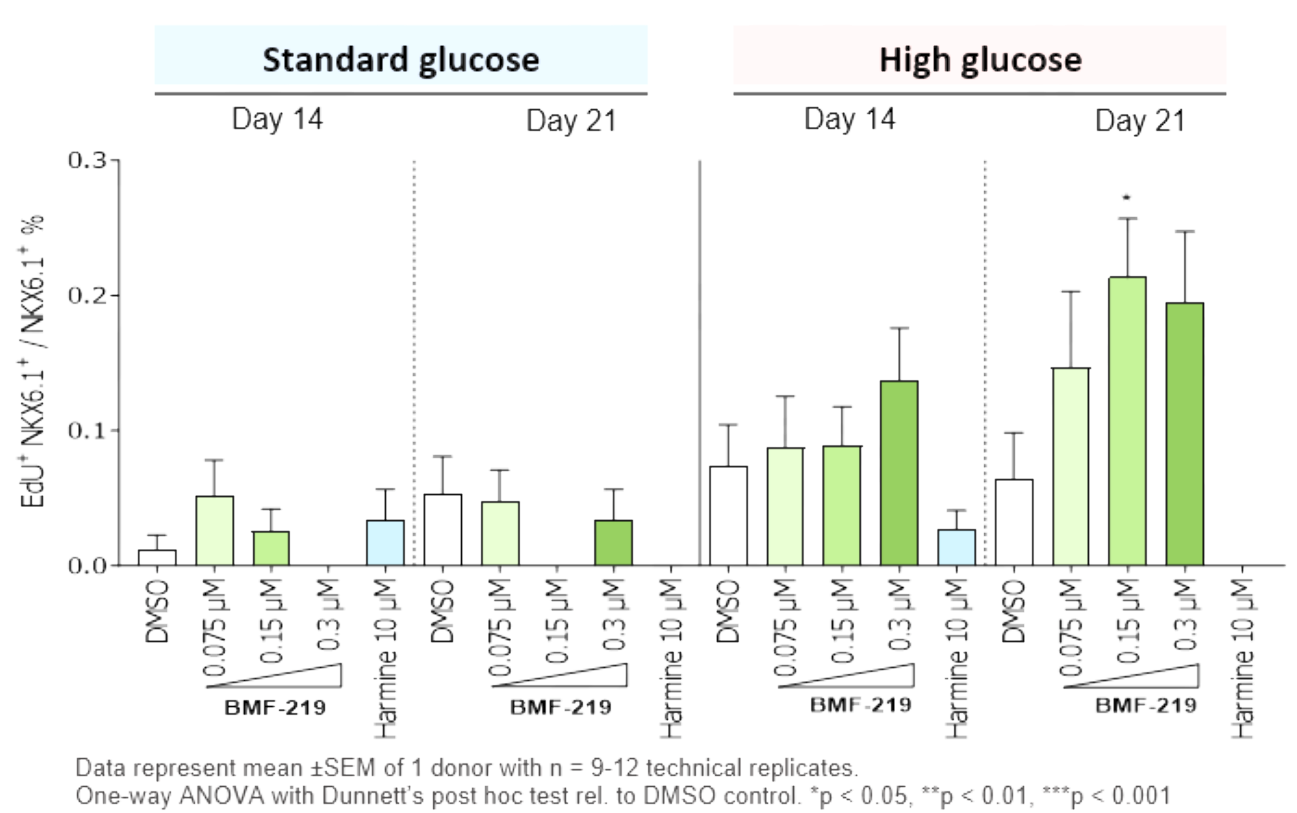
In addition in December 2023, preclinical ex vivo human islet data was presented. Dependent on dose concentration and also dependent on dose duration, BMF-219 was observed to increase beta-cell mass and function, as well as promote controlled proliferation and enhance insulin content in beta cells. Proliferation was observed only under elevated glucose conditions, which mimics diabetic levels, and with continuous drug exposure. BMF-219 was observed to upregulate the expression of key cell-cycle proteins, PbK and CCNA2 (Cyclin A2) in a glucose-dependent fashion. When not sequestered to menin, PbK expression was known to be upregulated by JunD, which is a glucose-sensitive menin binding partner. (Kulkarni et al. WCIRDC 2023)
Clinical Development
Biomea reported initial clinical data from the first two cohorts of the Phase 2 portion of COVALENT-111 (NCT05731544) in March of 2023. As reported, 89% of patients enrolled in Cohort 3 (n=10 patients at 100 mg without food) achieved a reduction in HbA1c, 78% achieved ≥ 0.5% reduction in HbA1c, and 56% achieved ≥ 1% reduction in HbA1c (median and mean reduction over the cohort: -1.0% and -0.81%, respectively). BMF-219 was well tolerated and demonstrated a favorable safety profile with no dose discontinuations.
Additional clinical data were presented in June of 2023 from the first two cohorts of patients with type 2 diabetes enrolled in the Phase 2 portion of COVALENT-111. This new data highlighted specific patients treated with BMF-219 for 4 weeks who maintained or experienced a further decrease in HbA1c levels 8 weeks after treatment was completed, up to a 2.4% reduction from baseline. (Rodriguez et al. ADA 2023)
Fig. Clinical data presented at 2023 ADA. The top 50% of responders after 4 weeks of treatment in Cohorts 2 and 3 (100 mg BMF-219 fed and fasted, respectively) demonstrated durable and ongoing reduction in HbA1c while off treatment up to Week 12; a continued reduction in HbA1c was observed in Cohort 2 (additional 114%) and in Cohort 3 (additional 62%) (Rodriguez et al. ADA 2023)
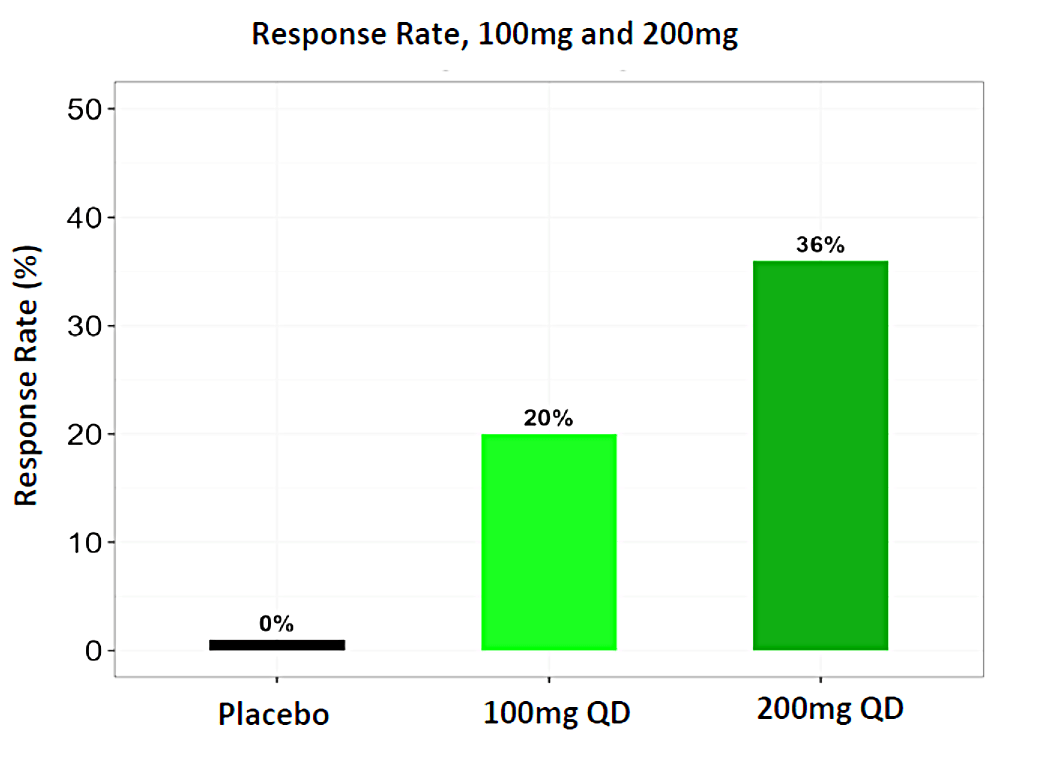
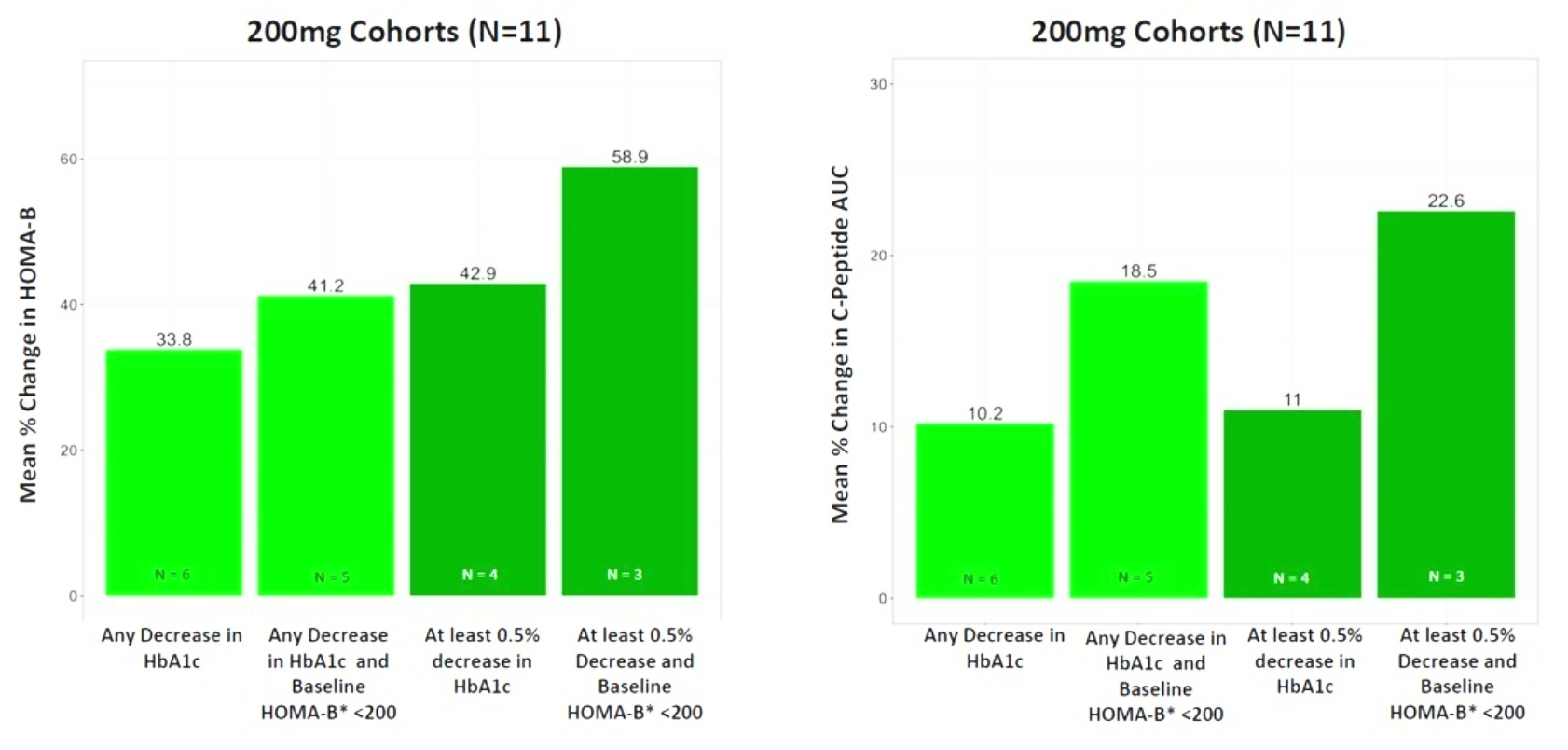
In December 2023, Biomea presented long-term follow-up data showing improved glycemic control after 22 weeks off treatment in an ongoing Phase 2 study (COVALENT-111) of BMF-219 in type 2 diabetes. At Week 26, 22 weeks after the last dose of BMF-219, participants in the 100 mg QD (without food) cohort saw an improved placebo-adjusted mean reduction in HbA1c of 0.8% (compared to a 0.7% placebo-adjusted mean reduction in HbA1c at Week 4). Observed HbA1C reduction was supported by an increase from baseline in placebo adjusted mean HOMA-B (+270%) and in mean stimulated C-peptide AUC (+22%) at Week 26 in responders (defined as HbA1c reduction ≥0.5% at Week 26) with baseline below the HOMA-B upper limit of normal (<200). BMF-219 was generally well tolerated; no dose reductions, dose discontinuations, or severe or serious adverse events, and no symptomatic or asymptomatic hypoglycemia was observed. In addition, Biomea reported that the 200 mg cohorts near doubled the percentage of patients (~36%) with durable HbA1c reduction of 1% or more compared to the 100 mg cohorts which reported earlier as 20%. (Frias et al. WCIRDC 2023)
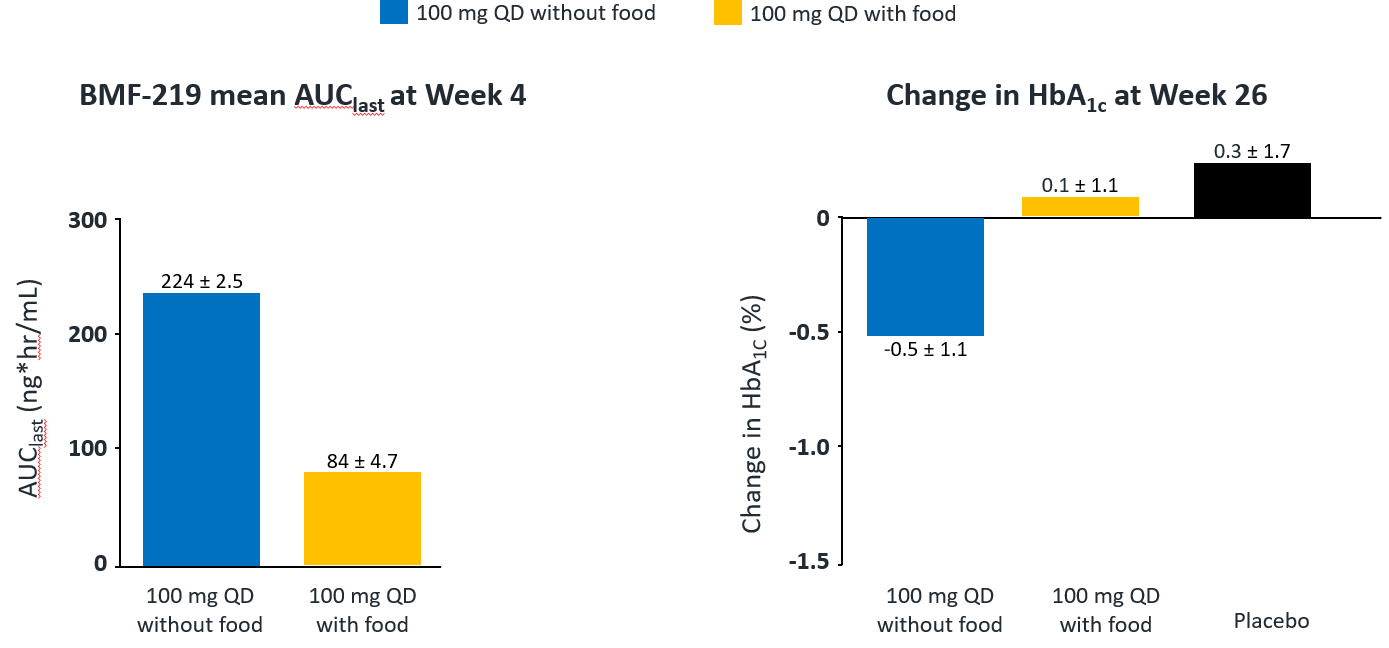
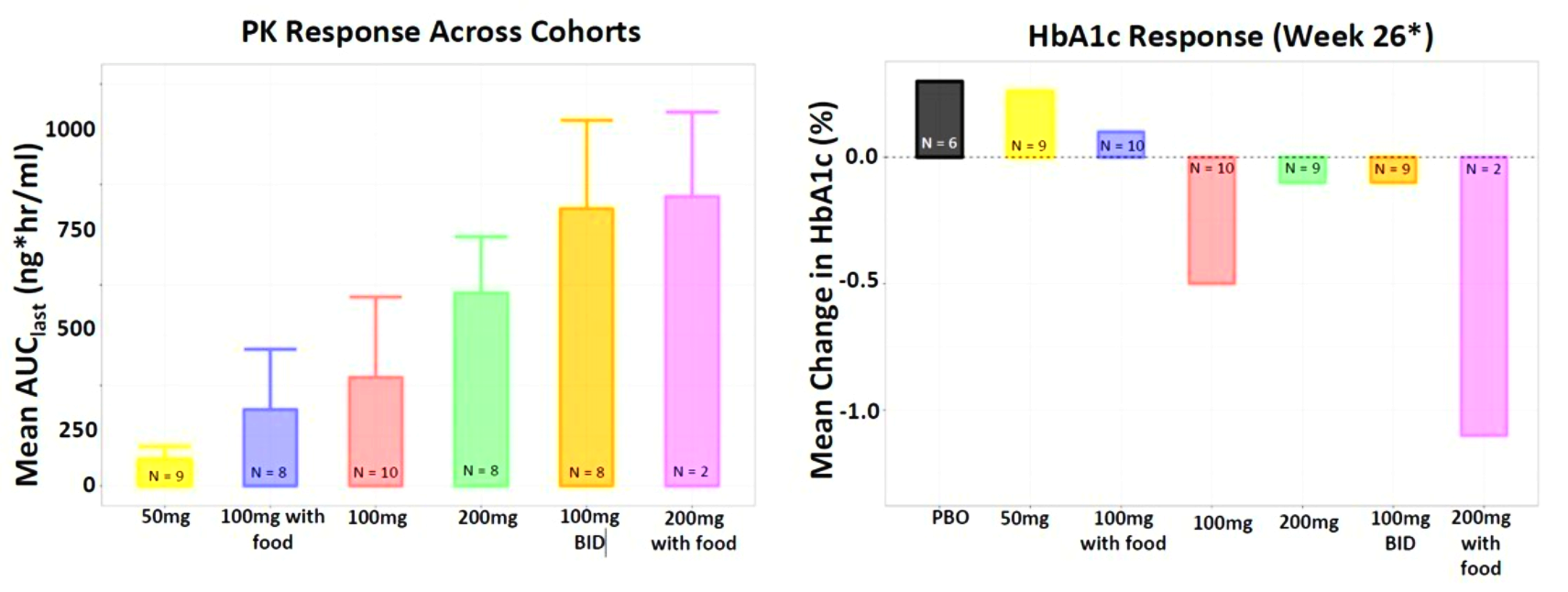
Fig. Clinical data presented at 2023 WCIRDC. Pharmacokinetics and HbA1c Response
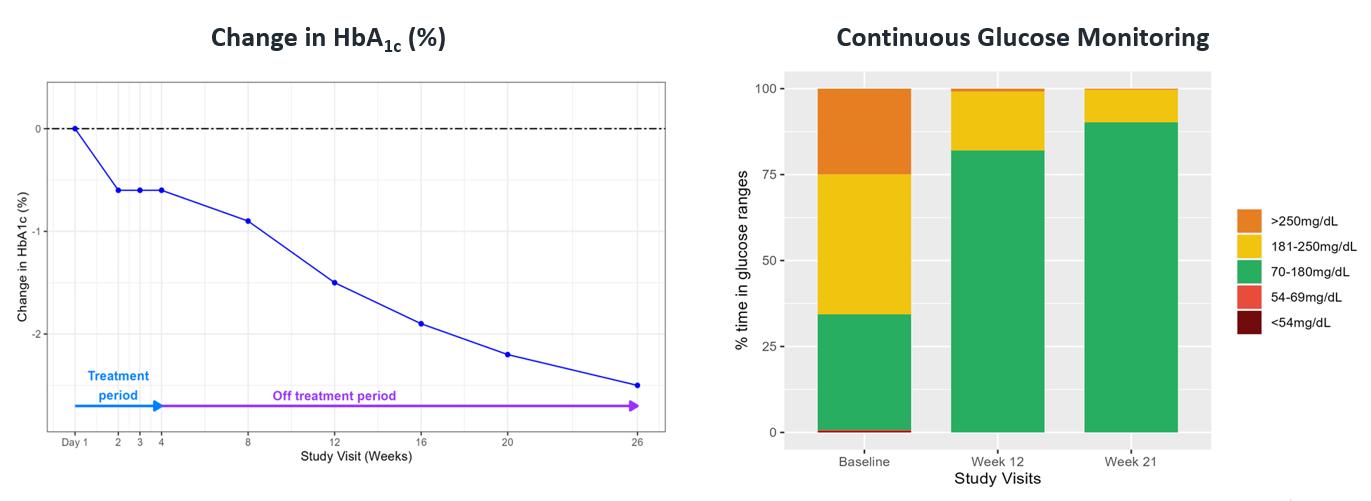
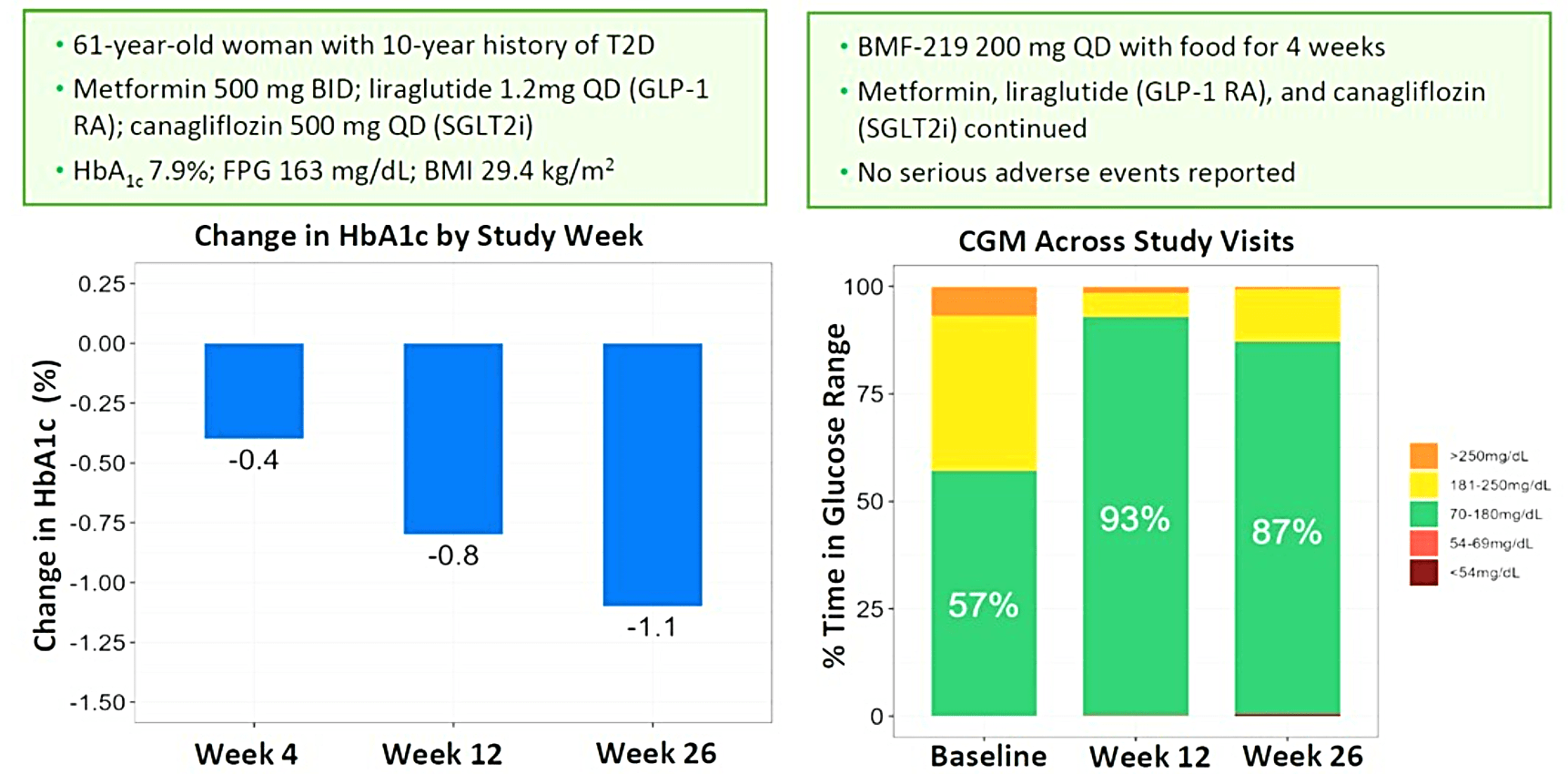
Fig. A case study of a 29-year-old man with 4-year history of T2D
Biomea presented additional clinical data sets from the dose escalation phase of COVALENT-111 in March of 2024, highlighting BMF-219’s novel mechanism of action in patients with type 2 diabetes. Patients in COVALENT-111 are displaying improved glycemic control while off therapy, supporting improved pancreatic function following BMF-219 treatment. Patients who demonstrated the greatest HbA1c reduction at Week 26 (22 weeks off treatment) had the greatest improvement in beta cell function as measured by HOMA-B and C-peptide. In patients failing current standard of care medications, at Week 26, following a 28-day dose cycle of BMF-219, a general dose response was observed with placebo-adjusted mean percent changes of HbA1c of -0.04% (50mg QD*), -0.2% (100mg QD with food), -0.8% (100mg), -0.4% (200mg QD), -0.4% (100mg BID), and -1.4% (200mg with food) (*50mg data out to Week 20, latest data cut). Across 100mg QD, 200mg QD, and 100mg BID cohorts (N=40), 38% of patients had ≥0.5% HbA1c reduction (with a mean HbA1c reduction of 1.2%), and 23% of patients had ≥1.0% HbA1c reduction (with a mean HbA1c reduction of 1.5%) at Week 26. Patients with >7 years duration of diabetes and failing dual- or triple-agent therapy (including GLP1 RA and/or SGLT2i) (n=2) also demonstrated improved glycemic control (HbA1c -0.4%, -1.1%, and -1.1% at Weeks 4, 12, and 26, respectively) with BMF-219 dosed at 200mg with food. Increase in HOMA-B and C-peptide generally correlated with glycemic control, consistent with BMF-219’s core mechanism of action: beta-cell proliferation and improved beta-cell function. BMF-219 was generally well tolerated with no serious adverse events and no adverse event-related study discontinuations, and no symptomatic or clinically significant hypoglycemia. 100mg and 200mg dose levels have been selected for the first 3 Arms of the Expansion Phase, which will dose patients up to 12 weeks (compared to 4 weeks in the Escalation Phase) and extended follow-up to Week 52. (Rodriguez et al. ATTD 2024, Abitbol et al. ATTD 2024, Denham et al. ATTD 2024)
Abstracts
Oral menin inhibitor, BMF-219, displays a significant and durable reduction in HbA1c in a type 2 diabetes rat model
Priyanka Somanath, Sanchita Mourya, Weiqun Li, Tenley C. Archer, Brian Law, Daniel Lu, Tripta Rughwani, Lekha Kumar, Taisei Kinoshita, Mini Balakrishnan, Thomas Butler;
EASD 2022 Short Oral Discussion (#590).
https://www.abstractsonline.com/pp8/#!/10613/presentation/1318
Oral long-acting menin inhibitor normalises type 2 diabetes in two rat models
Thomas Butler, Sanchita Mourya, Weiqun Li, Brian Law, Tenley Archer, Taisei Kinoshita, Priyanka Somanath
EASD 2022 Oral Presentation (#197).
https://www.abstractsonline.com/pp8/#!/10613/presentation/934
Oral Menin Inhibitor, BMF-219, Displays a Significant and Durable Reduction in HbA1c in a Type 2 Diabetes Mellitus Rat Model
Priyanka Somanath, Sanchita Mourya, Weiqun Li, Tenley C. Archer, Brian Law, Daniel Lu, Tripta Rughwani, Lekha Kumar, Taisei Kinoshita, Mini Balakrishnan, Thomas Butler
ADA Scientific Sessions 2022 (113-LB)
https://diabetesjournals.org/diabetes/article/71/Supplement_1/113-LB/146520/113-LB-Oral-Menin-Inhibitor-BMF-219-Displays-a
Oral Long-Acting Menin Inhibitor Normalizes Type 2 Diabetes Mellitus (T2DM) in Two Rat Models
Thomas Butler, Weiqun Li, Brian Law, Tenley Archer, Taisei Kinoshita, Priyanka Somanath
ADA Scientific Sessions 2022 (P-851)
https://diabetesjournals.org/diabetes/article/71/Supplement_1/851-P/145721/851-P-Oral-Long-Acting-Menin-Inhibitor-Normalizes
Literature References
Covalent Inhibition
Advances in covalent drug discovery
Lydia Boike, Nathaniel J. Henning, Daniel K Nomura
Nature Review Drug Discovery – 2022 Aug 25
https://pubmed.ncbi.nlm.nih.gov/36008483/
The ascension of targeted covalent inhibitors
Juswinder Singh
Journal of Medicinal Chemistry – 2022 Apr 19
https://pubmed.ncbi.nlm.nih.gov/36008483/
The design and development of covalent protein-protein interaction inhibitors for cancer treatment
Sha-sha Cheng, Gua-Jun Yang, Wanhe Wang, Chung-Hang Leung & Dik-Lung Ma
Journal of Hematology & Oncology – 2020 Mar 30
https://doi.org/10.1186/s13045-020-00850-0
Perspective on the kinetics of covalent and irreversible inhibition
John M. Strelow
SLAS Discovery – 2017 Jan
https://doi.org/10.1177/1087057116671509
Drug discovery for a new generation of covalent drugs
Amit S. Kalgutkar & Deepak K. Dalvie
Expert Opinion on Drug Discovery – 2012 May 19
https://doi.org/10.1517/17460441.2012.688744
Diabetes and Beta Cell Function
Type 2 diabetes-a matter of beta-cell life and death?
Christopher J. Rhodes
Science – 2005 Jan 21
307(5708):380-4. doi: 10.1126/science.1104345. PMID: 15662003.
https://pubmed.ncbi.nlm.nih.gov/15662003/
Diabetes Invest_Beta-cell failure in diabetes – Common susceptibility and mechanisms shared between type 1 and type 2 diabetes 1
Hiroshi Ikegami, Naru Babaya, and Shinsuke Noso
Journal of Diabetes Investigation – 2021 Sep;
12(9): 1526–1539. Published online 2021 Jun 16. doi: 10.1111/jdi.13576
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8409822/
Not control but conquest – Strategies for the remission of Type 2 diabetes mellitus
Jinyoung Kim, Hyuk-Sang Kwon
Diabetes and Metabolism Journal – 2022 Mar;
46(2):165-180. doi: 10.4093/dmj.2021.0377.
https://pubmed.ncbi.nlm.nih.gov/35385632/
Increased beta-cell proliferation before immune cell invasion prevents progression of Type 1 diabetes
Dirice E et al., 2019 Nature Metabolism
Nat Metab – 2019 May
1(5): 509–518. Published online 2019 May 6. doi: 10.1038/s42255-019-0061-8
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6696912/
Importance of beta cell mass for glycaemic control in people with Type 1 diabetes
Diabetologia, 2023 Feb
66(2):367-375. doi: 10.1007/s00125-022-05830-2. Epub 2022 Nov 17.
https://pubmed.ncbi.nlm.nih.gov/36394644/
Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes
International Journal of Molecular Science – 2016 May 17
17(5):744. doi: 10.3390/ijms17050744. PMID: 27196896; PMCID: PMC4881566.
https://pubmed.ncbi.nlm.nih.gov/27196896/
Remission of human Type 2 diabetes requires decrease on liver and pancreas fat content, but is dependent upon capacity for beta cell recovery
Cell Metabolism, 2018 Oct 2
28(4):547-556.e3. doi: 10.1016/j.cmet.2018.07.003.
https://pubmed.ncbi.nlm.nih.gov/30078554/
Intervention with therapeutic agents, Understanding the path to remission in Type 2 Diabetes: Part 1
Shuai Hao et al.
Endocrinology Metabolism Clinics of North America – 2023 Mar
52(1):27-38. doi: 10.1016/j.ecl.2022.07.003. Epub 2022 Nov 14.
https://pubmed.ncbi.nlm.nih.gov/36754495/
Intervention with therapeutic agents, Understanding the path to remission in Type 2 diabetes – Part 2
Shuai Hao et al.
Endocrinology Metabolism Clinics of North America – 2023 Mar
52(1):39-47. doi: 10.1016/j.ecl.2022.07.004. Epub 2022 Nov 18.
https://pubmed.ncbi.nlm.nih.gov/36754496/
Beta Cell Proliferation
Pancreatic β-cell proliferation in obesity
Linnemann AK, Baan M, Davis DB
Advances in Nutrition – 2014 May 14, 5(3):278-88. doi: 10.3945/an.113.005488. PMID: 24829474; PMCID: PMC4013180.
https://pubmed.ncbi.nlm.nih.gov/24829474/
A morphological study of the endocrine pancreas in human pregnancy
F.A. Van Assche FA, L. Aerts L, F. De Prins
British Journal of Obstet Gynaecology – 1978 Nov, 85(11):818-20. doi: 10.1111/j.1471-0528.1978.tb15835.x. PMID: 363135.
https://pubmed.ncbi.nlm.nih.gov/363135/
Beta cell adaptation to pregnancy requires prolactin action on both beta and non-beta cells
Shrivastava V, Lee M, Lee D, Pretorius M, Radford B, Makkar G, Huang C.
Scientific Reports – 2021 May 14, 11(1):10372. doi: 10.1038/s41598-021-89745-9. PMID: 33990661; PMCID: PMC8121891.
https://pubmed.ncbi.nlm.nih.gov/33990661
Prolactin-regulated Pbk is involved in pregnancy-induced β-cell proliferation in mice
Cao, Y., Feng, Z., He, X., Zhang, X., Xing, B., Wu, Y., Hojnacki, T., Katona, B. W., Ma, J., Zhan, X., & Hua, X. (2022)
Journal of Endocrinology, 252(2), 107-123.
https://doi.org/10.1530/JOE-21-0114
Expansion of β-cell mass in response to pregnancy
Sebastian Rieck and Klaus H. Kaestner
Trends in Endocrinology and Metabolism – 2010, Mar 21, (3):151-8. doi: 10.1016/j.tem.2009.11.001. Epub 2009 Dec 16.
https://pubmed.ncbi.nlm.nih.gov/20015659/
Beta-cell compensation and gestational diabetes
Taofeek O Usman, Goma Chhetri, Hsuan Yeh, H Henry Dong
Journal of Biological Chemistry – 2023, Dec, 299(12):105405. doi: 10.1016/j.jbc.2023.105405. Epub 2023 Oct 29.
https://pubmed.ncbi.nlm.nih.gov/38229396/
Serum from pregnant donors induces human beta cell proliferation and insulin secretion
Sylvester-Armstrong KR et al.
bioRxiv – 2023, Apr 17, 2023.04.17.537214. doi: 10.1101/2023.04.17.537214
https://pubmed.ncbi.nlm.nih.gov/37131658/
Modulatory role of prolactin in type 1 diabetes
Ramos-Martínez E et al.
Hormone Molecular Biology and Clinical Investigation – 2022, Jul 19, 44(1):79-88. doi: 10.1515/hmbci-2022-0008. eCollection 2023 Mar 1.
https://pubmed.ncbi.nlm.nih.gov/35852366/
Pancreatic islet cell type-specific transcriptomic changes during pregnancy and postpartum
Chung J-Y et al.
iScience – 2023 Mar 17, 26(4):106439. doi: 10.1016/j.isci.2023.106439. eCollection 2023 Apr 21.
https://pubmed.ncbi.nlm.nih.gov/37020962/
Breastfeeding can reduce the risk of developing diabetes
Soo Young Kim
Korean Journal of Family Medicine – 2018, 39(5):271-272. Published online: September 20, 2018 DOI: https://doi.org/10.4082/kjfm.39.5E
https://www.kjfm.or.kr/journal/view.php?number=4371
Potential protective effect of lactation against incidence of type 2 diabetes mellitus in women with previous gestational diabetes mellitus
Tanase-Nakao K et al.
Diabetes Metab Res Review – 2017 May, 33(4): e2875. Published online 2017 Feb 23. doi: 10.1002/dmrr.2875
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5434910/
Lactation and progression to type 2 diabetes in patients with gestational diabetes mellitus – A systematic review and meta-analysis of cohort studies
Feng L et al.
Journal of Diabetes Investigation – 2018 Nov 9, 9(6):1360-1369. doi: 10.1111/jdi.12838. Epub 2018 Apr 18.
https://pubmed.ncbi.nlm.nih.gov/29575786/
Lactation and progression to Type 2 diabetes mellitus after gestational diabetes mellitus – A prospective cohort study
Gunderson EP et al.
Annals of Internal Medicine – 2015 Dec 15, 163(12):889-98. doi: 10.7326/M15-0807. Epub 2015 Nov 24.
https://pubmed.ncbi.nlm.nih.gov/26595611/
Prior lactation reduces future diabetic risk sustained postweaning effects on insulin sensitivity
Bajaj H et al.
American Journal of Physiology: Endocrinology and Metabolism – 2017 Mar 1, 312(3):E215-E223. doi: 10.1152/ajpendo.00403.2016. Epub 2016 Dec 13.
https://pubmed.ncbi.nlm.nih.gov/27965206/
Association of lactation with maternal risk of type 2 diabetes – A systematic review and meta-analysis of observational studies
Pinho‐Gomes A-C et al.
Diabetes, Obesity and Metabolism – 2021 Aug, 23(8):1902-1916. doi: 10.1111/dom.14417. Epub 2021 May 20.
https://pubmed.ncbi.nlm.nih.gov/33908692/
Lactation duration and progression to diabetes in women across the childbearing years – The 30-year CARDIA study
Gunderson EP et al.
JAMA Internal Medicine – 2018 Mar, 178(3): 328–337. Published online 2018 Jan 16. doi: 10.1001/jamainternmed.2017.7978
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5885916/
Effect of breastfeeding and its duration on impaired fasting glucose and diabetes in perimenopausal and postmenopausal women – Korea National Health and Nutrition Examination Survey (KNHANES)
Kwan B-S et al.
Medicines – 2021 Nov 12, 8(11):71.doi: 10.3390/medicines8110071.
https://pubmed.ncbi.nlm.nih.gov/34822368/
Menin and Beta Cell Proliferation
Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c
Karnik, S. K., Hughes, C. M., Gu, X., Rozenblatt-Rosen, O., McLean, G. W., Xiong, Y., Meyerson, M., & Kim, S. K.
Proceedings of the National Academy of Sciences of the United States of America – 2005, 102(41), 14659–14664.
https://doi.org/10.1073/pnas.0503484102
Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene
Yang, Y., Gurung, B., Wu, T., Wang, H., Stoffers, D. A., & Hua, X.
Proceedings of the National Academy of Sciences of the United States of America – 2010, 107(47), 20358–20363.
https://doi.org/10.1073/pnas.1012257107
Deletion of the Men1 gene prevents streptozotocin-induced hyperglycemia in mice
Yang Y, Wang H, Hua X. .
Proceedings of the National Academy of the Sciences, US – 2011 Jan 17, 2010:876701. doi: 10.1155/2010/876701. Epub 2011 Jan 17. PMID: 21318185; PMCID: PMC3034935
https://pubmed.ncbi.nlm.nih.gov/21059956/
Glucose-mediated repression of menin promotes pancreatic β-cell proliferation
Zhang, H., Li, W., Wang, Q., Wang, X., Li, F., Zhang, C., Wu, L., Long, H., Liu, Y., Li, X., Luo, M., Li, G., & Ning, G. Endocrinology – 2012, 153(2), 602–611.
https://doi.org/10.1210/en.2011-1460
Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus
Karnik, S. K., Chen, H., McLean, G. W., Heit, J. J., Gu, X., Zhang, A. Y., Fontaine, M., Yen, M. H., & Kim, S. K.
Science (New York, N.Y.) – 2007, 318(5851), 806–809.
https://doi.org/10.1126/science.1146812
Menin-regulated Pbk controls high fat diet-induced compensatory beta cell proliferation
Ma, J., Xing, B., Cao, Y., He, X., Bennett, K. E., Tong, C., An, C., Hojnacki, T., Feng, Z., Deng, S., Ling, S., Xie, G., Wu, Y., Ren, Y., Yu, M., Katona, B. W., Li, H., Naji, A., & Hua, X.
EMBO molecular medicine – 2021, 13(5), e13524.
https://doi.org/10.15252/emmm.202013524
Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation.
Hughes, E., & Huang, C.
Endocrinology – 2011, 152(3), 847–855.
https://doi.org/10.1210/en.2010-1250
Combined inhibition of menin-MLL interaction and TGF-β signaling induces replication of human pancreatic beta cells
Pahlavanneshan S et al.
European Journal of Cell Biology – 2020 Jun;99(5):151094.doi: 10.1016/j.ejcb.2020.151094. Epub 2020 May 30.
https://pubmed.ncbi.nlm.nih.gov/32646642/
Induction of ß Cell Replication by Small Molecule-Mediated Menin Inhibition and Combined PKC Activation and TGF‑ß Inhibition as Revealed by A Refined Primary Culture Screening
Pahlavanneshan S et al.
Cell Journal – 2021 Nov;23(6):633-639.doi: 10.22074/cellj.2021.7437. Epub 2021 Nov 23.
https://pubmed.ncbi.nlm.nih.gov/34939756/
Epigenetic changes induced by high glucose in human pancreatic beta cells
Alhazzaa RA et al.
Journal of Diabetes Research – 2023 Feb 13:2023:9947294. doi: 10.1155/2023/9947294. eCollection 2023.
https://pubmed.ncbi.nlm.nih.gov/36815184/
VGLL4 and MENIN function as TEAD1 corepressors to block pancreatic β cell proliferation
Li F et al.
Cell Reports – 2023 Jan 31;42(1):111904.doi: 10.1016/j.celrep.2022.111904. Epub 2023 Jan 19.
https://pubmed.ncbi.nlm.nih.gov/36662616/
The potential of β‐cell growth promotion, continued
Zachary T. Bloomgarden
Journal of Diabetes – 2023 May; 15(5): 366–367. Published online 2023 Apr 27. doi: 10.1111/1753-0407.13396
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10172018/
Menin Science
Menin dynamics and functional insight: take your partners
Molecular and Cellular Biology – 2010 Sep 15;326(1-2):80-4.doi: 10.1016/j.mce.2010.04.011. Epub 2010 Apr 24.
https://pubmed.ncbi.nlm.nih.gov/20399832/
The role of menin in hematopoiesis
Ivan Maillard and Jay L. Hess
Advances in Experimental Medicine and Biology – 2009:668:51-7.doi: 10.1007/978-1-4419-1664-8_5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2981825/
Regulation cyclin B2 expression and cell cycle G2-M transition by menin
Ting Wu, Xiuli Zhang, Xiaohua Huang, Yuqing Yang,and Xianxin Hua
Journal of Biological Chemistry – 2010 Jun 11; 285(24): 18291–18300.
Published online 2010 Apr 19. doi: 10.1074/jbc.M110.106575
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2881754/
Endocrinology and Metabolism – 01 Sep 2011
https://doi.org/10.1152/ajpendo.00022.2011
A Review of the Scaffold Protein Menin and its Role in Hepatobiliary Pathology
Gene Expression –2017 Jul 7;17(3):251-263. doi: 10.3727/105221617X695744. Epub 2017 Apr 28.
https://pubmed.ncbi.nlm.nih.gov/28485270/
Epigenetic regulation by the menin pathway
Endocrine-related Cancer –2017 Oct;24(10):T147-T159. doi: 10.1530/ERC-17-0298. Epub 2017 Aug 15.
https://pubmed.ncbi.nlm.nih.gov/28811300/
Menin in Hematological Malignancies
Therapeutic implications of menin inhibition in acute leukemias
Issa, G. C., Ravandi, F., DiNardo, C. D., Jabbour, E., Kantarjian, H. M., & Andreeff, M.
Leukemia – 2021, 35(9), 2482–2495.
https://doi.org/10.1038/s41375-021-01309-y
Challenges and opportunities in targeting the menin–MLL interaction
Cierpicki, T., & Grembecka, J.
Future Medicinal Chemistry – 2014, 6(4), 447–462.
https://doi.org/10.4155/fmc.13.214
The Spectrum of MYC Alterations in Diffuse Large B-Cell Lymphoma
Xia, Y., & Zhang, X.
Acta haematologica – 2020, 143(6), 520–528.
https://doi.org/10.1159/000505892
Targeting MYC in multiple myeloma
Jovanović, K. K., Roche-Lestienne, C., Ghobrial, I. M., Facon, T., Quesnel, B., & Manier, S.
Leukemia – 2018, 32(6), 1295–1306.
https://doi.org/10.1038/s41375-018-0036-x
Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia
Kuhn, M. W. M., Song, E., Feng, Z., Sinha, A., Chen, C.-W., Deshpande, A. J., … Armstrong, S. A.
Cancer Discovery – 2016, 6(10), 1166–1181.
https://doi.org/10.1158/2159-8290.CD-16-0237
Menin in Solid Tumors
The same pocket in menin binds both MLL and JUND but has opposite effects on transcription
Jing Huang, Buddha Gurung, Bingbing Wan, Ke Wan, Xianxin Hua, and Ming Lei
Nature. 2012 Feb 12; 482(7386): 542–546. Published online 2012 Feb 12. doi: 10.1038/nature10806
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983792/
JunD, not c-Jun, is the AP-1 transcription factor required for Ras-induced lung cancer
JCI Insight – 2021 Jul 8;6(13):e124985. doi: 10.1172/jci.insight.124985.
https://pubmed.ncbi.nlm.nih.gov/34236045/
Loss of MLL Induces Epigenetic Dysregulation of Rasgrf1 to Attenuate Kras-Driven Lung Tumorigenesis
Cancer Research – 2022 Nov 15;82(22):4153-4163. doi: 10.1158/0008-5472.CAN-22-1475.
https://pubmed.ncbi.nlm.nih.gov/36098964/
Menin enhances c-Myc-mediated transcription to promote cancer progression. Nature communications
Nature Communications Vol 8, Article number: 15278 (2017)
https://www.nature.com/articles/ncomms15278
The scaffold protein menin is essential for activating the MYC locus and MYC-mediated androgen receptor transcription in androgen receptor-dependent prostate cancer cells
Cancer Communications (London, England) 2021 Dec;41(12):1427-1430. doi: 10.1002/cac2.12217. Epub 2021 Dec 1.
https://pubmed.ncbi.nlm.nih.gov/34850609/
