var prData = {"links":{"self":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news","first":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=1","next":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=2","prev":null,"last":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news?page=2"},"meta":{"executionDate":"2025-06-19T00:47:52","cmsDomain":"https://investors.biomeafusion.com","count":100},"data":[{"id":9576,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9576","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-preclinical-data-bmf-650-next-generation"},"title":"Biomea Fusion Reports Preclinical Data for BMF-650, a Next-Generation Oral GLP-1 Receptor Agonist Candidate, Demonstrating Robust Weight Loss and Appetite Suppression in Obese Non-Human Primates","type":{"title":"General","id":3886},"teaser":"Dose-dependent, marked reductions in food intake and significant weight loss observed in obese cynomolgus monkeys BMF-650 compared favorably to published data of a leading GLP-1 RA candidate IND filing on track for the second half of 2025; with Phase I study initiation in obese, otherwise healthy","language":"en","releaseDate":{"dateUTC":"2025-06-18T11:00:49","date":"2025-06-18T07:00:49","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Preclinical Data for BMF-650, a Next-Generation Oral GLP-1 Receptor Agonist Candidate, Demonstrating Robust Weight Loss and Appetite Suppression in Obese Non-Human Primates","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9576/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-06-18T11:00:55","lastUpdatedUTC":"2025-06-18T11:00:55"},{"id":9566,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9566","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-pricing-public-offering-securities"},"title":"Biomea Fusion Announces Pricing of Public Offering of Securities","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 17, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity company, today announced the pricing of its previously announced underwritten public offering consisting of (i) 19,450,000 shares of its common stock and","language":"en","releaseDate":{"dateUTC":"2025-06-18T03:15:51","date":"2025-06-17T23:15:51","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Pricing of Public Offering of Securities","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9566/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-06-18T03:15:55","lastUpdatedUTC":"2025-06-18T03:15:55"},{"id":9546,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9546","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-proposed-public-offering-securities"},"title":"Biomea Fusion Announces Proposed Public Offering of Securities","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 17, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity company, announced today that it has commenced an underwritten public offering of shares of its common stock and accompanying warrants to purchase shares of","language":"en","releaseDate":{"dateUTC":"2025-06-17T20:33:44","date":"2025-06-17T16:33:44","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Proposed Public Offering of Securities","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9546/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-06-17T20:33:52","lastUpdatedUTC":"2025-06-17T20:33:52"},{"id":9496,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9496","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-updated-preliminary-clinical-data"},"title":"Biomea Fusion Presents Updated Preliminary Clinical Data for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia at EHA 2025","type":{"title":"General","id":3886},"teaser":"New clinical results show sustained CRi, deep bone marrow responses, and encouraging survival in FLT3-mutant AML patients, including those previously treated with gilteritinib REDWOOD CITY, Calif., June 13, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a","language":"en","releaseDate":{"dateUTC":"2025-06-13T10:45:09","date":"2025-06-13T06:45:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Updated Preliminary Clinical Data for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia at EHA 2025","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9496/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-06-13T10:45:14","lastUpdatedUTC":"2025-06-13T10:45:14"},{"id":9486,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9486","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusions-bmf-500-selected-poster-presentation-eha-2025"},"title":"Biomea Fusion’s BMF-500 Selected for Poster Presentation at EHA 2025 Highlighting Phase I Data in Relapsed/Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., May 14, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity medicines company, today announced that preliminary clinical data from the Phase I COVALENT-103 trial of BMF-500 in adults with acute leukemia (AL) were","language":"en","releaseDate":{"dateUTC":"2025-05-14T20:05:49","date":"2025-05-14T16:05:49","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion’s BMF-500 Selected for Poster Presentation at EHA 2025 Highlighting Phase I Data in Relapsed/Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9486/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-05-14T20:05:59","lastUpdatedUTC":"2025-05-14T20:05:59"},{"id":9451,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9451","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2025-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2025 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Company Announces Strategic Realignment to Focus on Core Programs and Extend Cash Runway Icovamenib progressing toward the next phase of clinical development in insulin deficient type 2 diabetes patients and patients that are currently uncontrolled on a GLP-1 based therapy Biomea's next generation","language":"en","releaseDate":{"dateUTC":"2025-05-05T20:05:12","date":"2025-05-05T16:05:12","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2025 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9451/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-05-05T20:05:18","lastUpdatedUTC":"2025-05-05T20:05:18"},{"id":9426,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9426","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grants-under-nasdaq-1"},"title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., April 01, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity medicines company, today announced that on March 23, 2025, the compensation committee of Biomea’s board of directors granted two new","language":"en","releaseDate":{"dateUTC":"2025-04-01T20:01:29","date":"2025-04-01T16:01:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9426/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-04-01T20:01:31","lastUpdatedUTC":"2025-04-01T20:01:31"},{"id":9406,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9406","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2024"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Mick Hitchcock, Ph.D., appointed Interim Chief Executive Officer Biomea preparing icovamenib for late-stage clinical development Multiple milestones anticipated in 2025 including: FDA meeting anticipated in first half 2025 to discuss icovamenib late-stage development in severe insulin deficient","language":"en","releaseDate":{"dateUTC":"2025-03-31T20:10:30","date":"2025-03-31T16:10:30","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9406/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-03-31T20:10:38","lastUpdatedUTC":"2025-03-31T20:10:38"},{"id":9386,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9386","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-leadership-transition"},"title":"Biomea Fusion Announces Leadership Transition","type":{"title":"General","id":3886},"teaser":"
Board member Mick Hitchcock, named interim CEO replacing Thomas Butler. COO and President Ramses Erdtmann continuing at Biomea in current role. REDWOOD CITY, Calif., March 25, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea,” or the “Company”) (Nasdaq: BMEA), a clinical-stage diabetes and
","language":"en","releaseDate":{"dateUTC":"2025-03-25T23:00:29","date":"2025-03-25T19:00:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Leadership Transition","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9386/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-03-25T23:00:35","lastUpdatedUTC":"2025-03-25T23:19:07"},{"id":9351,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9351","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/icovamenib-treatment-patients-severe-insulin-deficient-diabetes"},"title":"Icovamenib Treatment in Patients with Severe Insulin-Deficient Diabetes Led to a Significant Improvement in Pancreatic Beta-cell Function with a 53% Mean Increase in C-peptide Levels 3 Months After Last Dose","type":{"title":"General","id":3886},"teaser":"
Therapeutic effects were sustained off treatment, with persistent reduction in HbA1c and improvement in beta-cell function 3 months after last dose, suggesting disease-modifying potential of icovamenib Strong correlation between C-peptide increase and HbA1c reduction (r = -0.73, p0.0001) across
","language":"en","releaseDate":{"dateUTC":"2025-03-24T12:30:12","date":"2025-03-24T08:30:12","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Icovamenib Treatment in Patients with Severe Insulin-Deficient Diabetes Led to a Significant Improvement in Pancreatic Beta-cell Function with a 53% Mean Increase in C-peptide Levels 3 Months After Last Dose","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9351/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-03-24T12:30:16","lastUpdatedUTC":"2025-03-24T19:53:33"},{"id":9311,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9311","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-3"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 03, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity medicines company, today announced that on March 3, 2025, the compensation committee of Biomea’s board of directors granted one new employee non-qualified","language":"en","releaseDate":{"dateUTC":"2025-03-03T13:00:14","date":"2025-03-03T08:00:14","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9311/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-03-03T13:00:20","lastUpdatedUTC":"2025-03-03T13:00:20"},{"id":9296,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9296","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-unveil-new-icovamenib-data-18th-international"},"title":"Biomea Fusion to Unveil New Icovamenib Data at the 18th International Conference on Advanced Technologies & Treatments for Diabetes","type":{"title":"General","id":3886},"teaser":"Presentation to highlight new clinical data from COVALENT-111, including c-peptide data REDWOOD CITY, Calif., Feb. 27, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity medicines company, today announced that it will feature two oral","language":"en","releaseDate":{"dateUTC":"2025-02-27T13:00:43","date":"2025-02-27T08:00:43","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Unveil New Icovamenib Data at the 18th International Conference on Advanced Technologies & Treatments for Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9296/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-02-27T13:00:45","lastUpdatedUTC":"2025-02-27T13:00:45"},{"id":9256,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9256","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-become-diabetes-obesity-medicines-company"},"title":"Biomea Fusion to Become a Diabetes & Obesity Medicines Company","type":{"title":"General","id":3886},"teaser":"Icovamenib & BMF-650 (oral small molecule GLP-1) are the cornerstones of the metabolic franchise Biomea preparing icovamenib for late-stage clinical development 2025 corporate update to be presented at the 43 rd Annual J.P. Morgan Healthcare Conference REDWOOD CITY, Calif., Jan.","language":"en","releaseDate":{"dateUTC":"2025-01-13T14:00:37","date":"2025-01-13T09:00:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Become a Diabetes & Obesity Medicines Company","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9256/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-13T14:00:41","lastUpdatedUTC":"2025-01-13T14:00:41"},{"id":9246,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9246","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-new-preclinical-data-icovamenib"},"title":"Biomea Fusion Reports New Preclinical Data on Icovamenib-Semaglutide Combination Study","type":{"title":"General","id":3886},"teaser":"New in vivo preclinical data announced today, demonstrate that icovamenib, in combination with semaglutide showed additional 11.5% body weight reduction and 43% increase in lean muscle mass compared to semaglutide alone Icovamenib, in combination with semaglutide, approximately doubled C-peptide","language":"en","releaseDate":{"dateUTC":"2025-01-07T14:00:58","date":"2025-01-07T09:00:58","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports New Preclinical Data on Icovamenib-Semaglutide Combination Study","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9246/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-07T14:01:00","lastUpdatedUTC":"2025-01-07T14:01:00"},{"id":9231,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9231","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-43rd-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at the 43rd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 06, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2025-01-06T12:00:37","date":"2025-01-06T07:00:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 43rd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9231/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2025-01-06T12:00:43","lastUpdatedUTC":"2025-01-06T12:00:43"},{"id":9211,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9211","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-positive-topline-results-ongoing-phase"},"title":"Biomea Fusion Announces Positive Topline Results from Ongoing Phase II COVALENT-111 Study in Patients with Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Icovamenib met the primary endpoint, displaying a meaningful statistically significant placebo-corrected mean reduction in HbA1c in the prespecified per protocol patient population Best response achieved in target, beta-cell deficient patients on one or more antidiabetic agents at baseline, showing","language":"en","releaseDate":{"dateUTC":"2024-12-17T13:10:38","date":"2024-12-17T08:10:38","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Positive Topline Results from Ongoing Phase II COVALENT-111 Study in Patients with Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9211/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-17T13:10:43","lastUpdatedUTC":"2024-12-17T13:10:43"},{"id":9201,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9201","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-announce-topline-results"},"title":"Biomea Fusion to Host Conference Call to Announce Topline Results from Phase II COVALENT-111 Study in Patients with Type 2 Diabetes (T2D)","type":{"title":"General","id":3886},"teaser":"Tuesday, December 17, 2024 at 8:00 am EST REDWOOD CITY, Calif., Dec. 16, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of","language":"en","releaseDate":{"dateUTC":"2024-12-16T23:16:01","date":"2024-12-16T18:16:01","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call to Announce Topline Results from Phase II COVALENT-111 Study in Patients with Type 2 Diabetes (T2D)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9201/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-16T23:16:04","lastUpdatedUTC":"2024-12-16T23:16:04"},{"id":9166,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9166","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-oral-and-poster-presentations-icovamenib"},"title":"Biomea Fusion Announces Oral and Poster Presentations of Icovamenib at the 22nd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"In preclinical experiments, icovamenib enhanced beta cell function and responsiveness of human islets to GLP-1-based therapies. These effects were associated with an increase in the expression levels of both the GLP-1 receptor (GLP-1R) as well as intracellular insulin.","language":"en","releaseDate":{"dateUTC":"2024-12-12T22:02:59","date":"2024-12-12T17:02:59","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Oral and Poster Presentations of Icovamenib at the 22nd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9166/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-12T22:03:04","lastUpdatedUTC":"2024-12-12T22:03:04"},{"id":9156,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9156","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-preliminary-data-ongoing-covalent-103"},"title":"Biomea Fusion Announces Preliminary Data from Ongoing COVALENT-103 Study of Investigational Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"Preliminary data supports BMF-500’s potential as a transformative therapy for patients with FLT3 mutated relapsed or refractory (R/R) acute leukemia BMF-500 showed a favorable safety and tolerability profile, with no dose-limiting toxicities observed across all dose levels Pharmacokinetic and","language":"en","releaseDate":{"dateUTC":"2024-12-09T21:01:30","date":"2024-12-09T16:01:30","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Preliminary Data from Ongoing COVALENT-103 Study of Investigational Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9156/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-09T21:01:39","lastUpdatedUTC":"2024-12-09T21:01:39"},{"id":9136,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9136","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-present-initial-clinical-data"},"title":"Biomea Fusion to Host Conference Call to Present initial Clinical Data from Phase I COVALENT-103 Study of BMF-500, a Covalent FLT3 Inhibitor, in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Dec. 06, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2024-12-06T13:00:32","date":"2024-12-06T08:00:32","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call to Present initial Clinical Data from Phase I COVALENT-103 Study of BMF-500, a Covalent FLT3 Inhibitor, in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9136/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-06T13:00:34","lastUpdatedUTC":"2024-12-06T13:00:34"},{"id":9121,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9121","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-2"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Dec. 02, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of oral covalent small molecules to treat and improve the lives of patients with diabetes,","language":"en","releaseDate":{"dateUTC":"2024-12-02T21:05:31","date":"2024-12-02T16:05:31","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9121/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-12-02T21:05:36","lastUpdatedUTC":"2024-12-02T21:05:36"},{"id":9056,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9056","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/late-breaker-oral-presentation-showing-new-analysis-escalation"},"title":"Late-Breaker Oral Presentation Showing New Analysis from the Escalation Portion of COVALENT-111 Presented at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","type":{"title":"General","id":3886},"teaser":"Icovamenib Achieves a Mean Reduction in HbA1c Greater than 1% at Week 26 Following 4 Weeks of Dosing in Type 2 Diabetes Patients Characterized by Insulin Deficiency 32 patients from the 100mg and 200mg cohorts, doses selected for the expansion phase, were characterized based on baseline biomarkers","language":"en","releaseDate":{"dateUTC":"2024-11-18T13:00:29","date":"2024-11-18T08:00:29","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Late-Breaker Oral Presentation Showing New Analysis from the Escalation Portion of COVALENT-111 Presented at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9056/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-11-18T13:00:36","lastUpdatedUTC":"2024-11-18T13:00:36"},{"id":9041,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9041","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-1"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Nov. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of oral covalent small molecules to treat and improve the lives of patients with diabetes,","language":"en","releaseDate":{"dateUTC":"2024-11-01T20:06:18","date":"2024-11-01T16:06:18","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9041/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-11-01T20:06:25","lastUpdatedUTC":"2024-11-01T20:06:25"},{"id":9016,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9016","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-preclinical-data-showing-icovamenib-bmf"},"title":"Biomea Fusion Presents Preclinical Data Showing Icovamenib (BMF-219) Enhanced Effectiveness of GLP-1-Based Therapies and Introduces BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist Candidate","type":{"title":"General","id":3886},"teaser":"Preclinical data from ex vivo human islet experiments showed that icovamenib (BMF-219) was able to enhance the activity of glucagon-like peptide-1 (GLP-1)-based therapies, potentially leading to increased insulin secretion and improved glycemic control in patients with diabetes Phase II study","language":"en","releaseDate":{"dateUTC":"2024-10-30T20:01:10","date":"2024-10-30T16:01:10","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Preclinical Data Showing Icovamenib (BMF-219) Enhanced Effectiveness of GLP-1-Based Therapies and Introduces BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist Candidate","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/9016/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-30T20:01:21","lastUpdatedUTC":"2024-10-30T20:01:21"},{"id":8996,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8996","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"U.S. Food and Drug Administration (FDA) lifted Clinical Hold on COVALENT-111 (Type 2 Diabetes) & COVALENT-112 (Type 1 Diabetes) trials COVALENT-111 Phase 2b 26-week topline data of the dose expansion cohorts expected in December 2024 COVALENT-112 Phase 2a 26-week topline data of the open label","language":"en","releaseDate":{"dateUTC":"2024-10-29T20:46:46","date":"2024-10-29T16:46:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8996/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-29T20:46:51","lastUpdatedUTC":"2024-10-29T20:46:51"},{"id":8981,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8981","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-approval-icovamenib-international"},"title":"Biomea Fusion Announces Approval of “icovamenib” as International Nonproprietary Name for BMF-219","type":{"title":"General","id":3886},"teaser":"Icovamenib is an oral covalent menin inhibitor in clinical development to investigate its impact on the function of insulin-producing beta cells REDWOOD CITY, Calif., Oct. 21, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company","language":"en","releaseDate":{"dateUTC":"2024-10-21T13:00:57","date":"2024-10-21T09:00:57","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Approval of “icovamenib” as International Nonproprietary Name for BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8981/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-21T13:01:01","lastUpdatedUTC":"2024-10-21T13:01:01"},{"id":8971,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8971","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-and-webcast-wednesday-october"},"title":"Biomea Fusion to Host Conference Call and Webcast on Wednesday, October 30th at 4:30 pm ET to Announce Our Lead Clinical Candidate, BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist and Preclinical Data Combining BMF-219 with a GLP-1 RA-Based Therapy","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Oct. 15, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with diabetes, obesity, and genetically","language":"en","releaseDate":{"dateUTC":"2024-10-15T20:05:48","date":"2024-10-15T16:05:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call and Webcast on Wednesday, October 30th at 4:30 pm ET to Announce Our Lead Clinical Candidate, BMF-650, a Next-Generation, Oral Small-Molecule GLP-1 Receptor Agonist and Preclinical Data Combining BMF-219 with a GLP-1 RA-Based Therapy","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8971/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-15T20:05:57","lastUpdatedUTC":"2024-10-15T20:05:57"},{"id":8956,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8956","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-1st-annual-asian-conference-innovative"},"title":"Biomea Fusion to Present at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational oral covalent menin inhibitor developed to regenerate insulin-producing beta cells Two trial-in-progress oral presentations to feature Phase II study design of oral covalent menin inhibitor BMF-219 in patients with type 2 diabetes (COVALENT-111) and in patients with","language":"en","releaseDate":{"dateUTC":"2024-10-07T13:01:09","date":"2024-10-07T09:01:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 1st Annual Asian Conference on Innovative Therapies for Diabetes Management (ATTD-ASIA 2024)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8956/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-07T13:01:11","lastUpdatedUTC":"2024-10-07T13:01:11"},{"id":8931,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8931","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-announces-formation-global-scientific-advisory-board-22"},"title":"Biomea Announces Formation of Global Scientific Advisory Board with 22 World-Renowned Diabetes Experts","type":{"title":"General","id":3886},"teaser":"International diabetes pioneers and thought leaders from 11 countries will work with Biomea leadership to unlock the potential of menin science and beta cell biology to advance BMF-219 and Biomea’s evolving pipeline REDWOOD CITY, Calif., Oct. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2024-10-01T13:00:29","date":"2024-10-01T09:00:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Announces Formation of Global Scientific Advisory Board with 22 World-Renowned Diabetes Experts","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8931/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-10-01T13:00:39","lastUpdatedUTC":"2024-10-01T16:58:29"},{"id":8921,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8921","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/fda-lifts-clinical-hold-bmf-219-type-2-and-type-1-diabetes"},"title":"FDA Lifts Clinical Hold on BMF-219 in Type 2 and Type 1 Diabetes Trials","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Sept. 26, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to treat and improve the lives of patients with metabolic","language":"en","releaseDate":{"dateUTC":"2024-09-26T18:01:15","date":"2024-09-26T14:01:15","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"FDA Lifts Clinical Hold on BMF-219 in Type 2 and Type 1 Diabetes Trials","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8921/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-09-26T18:01:25","lastUpdatedUTC":"2024-09-26T18:01:25"},{"id":8806,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8806","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-0"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Aug. 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with metabolic diseases and genetically defined","language":"en","releaseDate":{"dateUTC":"2024-08-01T20:05:36","date":"2024-08-01T16:05:36","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8806/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-08-01T20:05:44","lastUpdatedUTC":"2024-08-01T20:05:44"},{"id":8791,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8791","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"COVALENT-111 Phase 2b on track for Q4 2024 readout COVALENT-112 Phase 2a on track for Q4 2024 readout Announcement of the third program in obesity on track for Q3 2024 REDWOOD CITY, Calif., July 31, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or “the Company”) (Nasdaq: BMEA), a","language":"en","releaseDate":{"dateUTC":"2024-07-31T20:06:04","date":"2024-07-31T16:06:04","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8791/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-07-31T20:06:13","lastUpdatedUTC":"2024-07-31T20:06:13"},{"id":8721,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8721","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-bmf-219-diabetes-placed-clinical-hold"},"title":"Biomea Fusion Announces BMF-219 in Diabetes Placed on Clinical Hold ","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 06, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), announced that the Company has received notice from the U.S. Food and Drug Administration (FDA) that a full clinical hold has been placed on Biomea’s ongoing Phase I/II clinical","language":"en","releaseDate":{"dateUTC":"2024-06-06T20:06:41","date":"2024-06-06T16:06:41","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces BMF-219 in Diabetes Placed on Clinical Hold ","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8721/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-06-06T20:06:51","lastUpdatedUTC":"2024-06-06T20:06:51"},{"id":8711,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8711","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grant-under-nasdaq-listing"},"title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 03, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-06-03T20:05:58","date":"2024-06-03T16:05:58","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grant under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8711/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-06-03T20:06:04","lastUpdatedUTC":"2024-06-03T20:06:04"},{"id":8701,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8701","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-completion-enrollment-first-3-arms-phase"},"title":"Biomea Fusion Announces Completion of Enrollment of First 3 Arms in Phase 2 Expansion Cohorts of COVALENT-111 Study for BMF-219 in Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells REDWOOD CITY, Calif., May 30, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing","language":"en","releaseDate":{"dateUTC":"2024-05-30T13:12:58","date":"2024-05-30T09:12:58","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Completion of Enrollment of First 3 Arms in Phase 2 Expansion Cohorts of COVALENT-111 Study for BMF-219 in Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8701/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-05-30T13:13:07","lastUpdatedUTC":"2024-05-30T13:13:07"},{"id":8671,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8671","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2024-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2024 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported positive data from the escalation portion of Phase 1/2 study (COVALENT-111) in type 2 diabetes patients, displaying durable improved glycemic control while off therapy for 22 weeks, supporting the disease-modifying potential of BMF-219 to address a root cause of diabetes: a loss of","language":"en","releaseDate":{"dateUTC":"2024-05-02T20:05:27","date":"2024-05-02T16:05:27","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2024 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8671/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-05-02T20:05:36","lastUpdatedUTC":"2024-05-02T20:05:36"},{"id":8631,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8631","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grants-under-nasdaq-0"},"title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., April 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-04-01T20:11:09","date":"2024-04-01T16:11:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8631/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T20:11:11","lastUpdatedUTC":"2024-04-01T20:11:11"},{"id":8616,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8616","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-highlights-initial-data-first-two-type-1-diabetes"},"title":"Biomea Fusion Highlights Initial Data from the First Two Type 1 Diabetes Patients Dosed with BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes The first two type 1 diabetes patients enrolled in COVALENT-112 both demonstrated early signs of clinical activity with improved measures of beta-cell","language":"en","releaseDate":{"dateUTC":"2024-04-01T13:02:44","date":"2024-04-01T09:02:44","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Highlights Initial Data from the First Two Type 1 Diabetes Patients Dosed with BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8616/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T13:02:53","lastUpdatedUTC":"2024-04-01T13:02:53"},{"id":8601,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8601","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2023"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"In 2023, reported Phase 2 data (COVALENT-111) in type 2 diabetes patients supporting the disease-modifying potential of BMF-219 to address a root cause of diabetes: a loss of healthy, insulin-producing beta cells. After just a 4-week treatment period in type 2 diabetes patients, who had previously","language":"en","releaseDate":{"dateUTC":"2024-04-01T13:00:37","date":"2024-04-01T09:00:37","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8601/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-04-01T13:00:43","lastUpdatedUTC":"2024-04-01T13:00:43"},{"id":8581,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8581","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-patient-cohorts-covalent-111-displaying"},"title":"Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function","type":{"title":"General","id":3886},"teaser":"Three Clinical Data Sets from the Dose Escalation Phase of COVALENT-111 to be Presented at the 17 th Annual ATTD Conference Highlighting BMF-219’s Novel Mechanism of Action in Patients with Type 2 Diabetes Patients in COVALENT-111 are displaying improved glycemic control while off therapy out to","language":"en","releaseDate":{"dateUTC":"2024-03-06T08:05:37","date":"2024-03-06T03:05:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8581/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-03-06T08:05:45","lastUpdatedUTC":"2024-03-06T08:05:45"},{"id":8551,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8551","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-inc-reports-inducement-grants-under-nasdaq-listing"},"title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 01, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA) (“Biomea” or the “Company”), a clinical stage biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2024-03-01T21:16:53","date":"2024-03-01T16:16:53","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion, Inc. Reports Inducement Grants under Nasdaq Listing Rule 5635(c)(4)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8551/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-03-01T21:16:56","lastUpdatedUTC":"2024-03-01T21:16:56"},{"id":8451,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8451","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-highlights-recent-updates-and-anticipated-2024"},"title":"Biomea Fusion Highlights Recent Updates and Anticipated 2024 Corporate Milestones at 42nd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"Expansion portion of Phase II COVALENT-111 study readout in 216 patients with type 2 diabetes expected in 2024. Open label portion of Phase II COVALENT-112 study readout in 40 patients with type 1 diabetes expected in 2024. REDWOOD CITY, Calif., Jan. 09, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2024-01-09T16:03:07","date":"2024-01-09T11:03:07","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Highlights Recent Updates and Anticipated 2024 Corporate Milestones at 42nd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8451/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-09T16:03:08","lastUpdatedUTC":"2024-01-09T16:03:08"},{"id":8446,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8446","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-type-1-diabetes-patient"},"title":"Biomea Fusion Announces Dosing of First Type 1 Diabetes Patient in Phase II Study (COVALENT-112) with BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes The clinical study, COVALENT-112, has initiated enrollment of adults living with type 1 diabetes in the US and Canada.","language":"en","releaseDate":{"dateUTC":"2024-01-08T14:01:49","date":"2024-01-08T09:01:49","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Type 1 Diabetes Patient in Phase II Study (COVALENT-112) with BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8446/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-08T14:01:55","lastUpdatedUTC":"2024-01-08T14:01:55"},{"id":8421,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8421","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-42nd-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at the 42nd Annual J.P. Morgan Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 02, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2024-01-02T21:05:37","date":"2024-01-02T16:05:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at the 42nd Annual J.P. Morgan Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8421/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2024-01-02T21:05:45","lastUpdatedUTC":"2024-01-02T21:05:45"},{"id":8396,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8396","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-achievement-minimal-residual-disease"},"title":"Biomea Fusion Presents Achievement of Minimal Residual Disease Negativity (MRD-neg) in First Complete Responder from Ongoing Phase I Study (COVALENT-101) of BMF-219 in Patients with Relapsed or Refractory (R/R) Acute Myeloid Leukemia (AML) at the 2023 ASH","type":{"title":"General","id":3886},"teaser":"BMF-219 demonstrated early signs of clinical activity and ability to achieve durable and sustained complete responses (CRs) with minimal residual disease negativity (MRD-neg) in acute myeloid leukemia (AML) patients Pharmacodynamic data further supports the mechanism of action of BMF-219 as a menin","language":"en","releaseDate":{"dateUTC":"2023-12-11T14:25:47","date":"2023-12-11T09:25:47","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Achievement of Minimal Residual Disease Negativity (MRD-neg) in First Complete Responder from Ongoing Phase I Study (COVALENT-101) of BMF-219 in Patients with Relapsed or Refractory (R/R) Acute Myeloid Leukemia (AML) at the 2023 ASH","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8396/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-11T14:25:55","lastUpdatedUTC":"2023-12-11T14:25:55"},{"id":8376,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8376","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-near-doubling-percentage-patients"},"title":"Biomea Fusion Announces Near Doubling the Percentage of Patients with Durable HbA1c Reduction in the 200 mg Dose Cohorts","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes At Week 26, 22 weeks after the last dose of BMF-219, the 200 mg cohorts increased the percentage of patients to approximately 40% with durable HbA1c","language":"en","releaseDate":{"dateUTC":"2023-12-09T22:30:45","date":"2023-12-09T17:30:45","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Near Doubling the Percentage of Patients with Durable HbA1c Reduction in the 200 mg Dose Cohorts","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8376/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-09T22:30:55","lastUpdatedUTC":"2023-12-09T22:30:55"},{"id":8356,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8356","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-long-term-follow-data-showing-improved"},"title":"Biomea Fusion Presents Long-Term Follow-Up Data Showing Improved Glycemic Control after 22 Weeks Off Treatment in Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes in a Poster Presentation at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes At Week 26, 22 weeks after the last dose of BMF-219, participants in the 100 mg QD (without food) cohort saw an improved placebo adjusted mean reduction in","language":"en","releaseDate":{"dateUTC":"2023-12-08T14:00:12","date":"2023-12-08T09:00:12","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Long-Term Follow-Up Data Showing Improved Glycemic Control after 22 Weeks Off Treatment in Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes in a Poster Presentation at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8356/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-08T14:00:16","lastUpdatedUTC":"2023-12-08T14:12:32"},{"id":8346,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8346","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-long-term-follow-data-ongoing-phase-ii"},"title":"Biomea Fusion to Present Long-Term Follow-Up Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes and Results from Ex-Vivo Human Islet Experiments at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes In a poster presentation on Thursday, Dec. 7 th at 6:30pm and an oral presentation on Friday, Dec. 8 th at 7:30pm, Biomea to present new preclinical and","language":"en","releaseDate":{"dateUTC":"2023-12-07T14:15:42","date":"2023-12-07T09:15:42","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Long-Term Follow-Up Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Adults with Type 2 Diabetes and Results from Ex-Vivo Human Islet Experiments at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8346/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-07T14:15:45","lastUpdatedUTC":"2023-12-07T17:50:25"},{"id":8331,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8331","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-health-canada-clearance-clinical-trial"},"title":"Biomea Fusion Announces Health Canada Clearance of Clinical Trial Application (CTA) for BMF-219 in Type 1 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is a novel investigational covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes Health Canada has cleared the initiation of COVALENT-112, a Phase II clinical trial of BMF-219 in adults living with type 1 diabetes (T1D), following FDA","language":"en","releaseDate":{"dateUTC":"2023-12-05T13:31:10","date":"2023-12-05T08:31:10","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Health Canada Clearance of Clinical Trial Application (CTA) for BMF-219 in Type 1 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8331/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-12-05T13:31:16","lastUpdatedUTC":"2023-12-05T13:31:16"},{"id":8286,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8286","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-acceptance-three-abstracts-its-novel"},"title":"Biomea Fusion Announces Acceptance of Three Abstracts for its Novel Menin Inhibitor Highlighting Durable Glycemic Control in Diabetes Patients at the International Conference on Advanced Technologies & Treatments for Diabetes (ATTD) in 2024","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor developed to regenerate insulin-producing beta cells with the aim to cure diabetes New clinical data from all dosing cohorts (n=62) initiated to date from the escalation portion of COVALENT-111 and long-term (26 weeks) follow-up data of","language":"en","releaseDate":{"dateUTC":"2023-11-27T13:31:19","date":"2023-11-27T08:31:19","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Acceptance of Three Abstracts for its Novel Menin Inhibitor Highlighting Durable Glycemic Control in Diabetes Patients at the International Conference on Advanced Technologies & Treatments for Diabetes (ATTD) in 2024","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8286/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-27T13:31:27","lastUpdatedUTC":"2023-11-27T13:31:27"},{"id":8276,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8276","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-abstract-bmf-219-novel-therapeutic-agent"},"title":"Biomea Fusion Abstract “BMF-219: A Novel Therapeutic Agent to Reestablish Functional Beta Cells and Provide Long-term Glycemic Control\" Selected as One of Six Oral Presentations at 21st World Congress Insulin Resistance, Diabetes & Cardiovascular Disease","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure diabetes New clinical data providing long-term 26 weeks follow-up of the initial two dosing cohorts of COVALENT-111 will be presented during the Abstract Oral","language":"en","releaseDate":{"dateUTC":"2023-11-16T13:31:35","date":"2023-11-16T08:31:35","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Abstract “BMF-219: A Novel Therapeutic Agent to Reestablish Functional Beta Cells and Provide Long-term Glycemic Control\" Selected as One of Six Oral Presentations at 21st World Congress Insulin Resistance, Diabetes & Cardiovascular Disease","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8276/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-16T13:31:39","lastUpdatedUTC":"2023-11-16T13:31:39"},{"id":8266,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8266","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-data-ongoing-phase-ii-study-covalent"},"title":"Biomea Fusion to Present New Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","type":{"title":"General","id":3886},"teaser":"BMF-219 is an investigational novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure type 2 diabetes An abstract, “BMF-219: A novel therapeutic agent to reestablish functional beta cells and provide long-term glycemic control” will be presented","language":"en","releaseDate":{"dateUTC":"2023-11-08T21:08:36","date":"2023-11-08T16:08:36","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Data from Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes at the World Congress Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8266/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-08T21:08:50","lastUpdatedUTC":"2023-11-08T21:08:50"},{"id":8256,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8256","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-two-poster-presentations-upcoming-ash"},"title":"Biomea Fusion Announces Two Poster Presentations at Upcoming ASH Annual Meeting 2023","type":{"title":"General","id":3886},"teaser":"First presentation of clinical data from ongoing COVALENT-101 trial of covalent menin inhibitor BMF-219 as a treatment for patients with liquid tumors Trial in progress presentation featuring study design of ongoing COVALENT-103 trial of covalent FLT3 inhibitor BMF-500 REDWOOD CITY, Calif., Nov.","language":"en","releaseDate":{"dateUTC":"2023-11-02T13:06:51","date":"2023-11-02T09:06:51","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Two Poster Presentations at Upcoming ASH Annual Meeting 2023","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8256/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-11-02T13:06:57","lastUpdatedUTC":"2023-11-02T13:06:57"},{"id":8231,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8231","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Demonstrated durable HbA1c lowering in the escalation portion of ongoing Phase II study in type 2 diabetes (COVALENT-111), with 84% of all patients showing a reduction of HbA1c after 4 weeks dosing and 74% after another 8 weeks off-treatment period Expansion portion of COVALENT-111 cleared and","language":"en","releaseDate":{"dateUTC":"2023-10-30T20:01:48","date":"2023-10-30T16:01:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8231/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-30T20:01:57","lastUpdatedUTC":"2023-10-30T20:01:57"},{"id":8226,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8226","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-covalent-flt3"},"title":"Biomea Fusion Announces First Patient Dosed with Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia in Phase I Clinical Trial (COVALENT-103)","type":{"title":"General","id":3886},"teaser":"BMF-500, a novel 3 rd generation oral covalent inhibitor of FMS-like tyrosine kinase 3 (FLT3), is the second product candidate discovered and developed by Biomea’s proprietary FUSION™ System to enter the clinic Phase I study (COVALENT-103) of BMF-500 will examine monotherapy safety and efficacy in","language":"en","releaseDate":{"dateUTC":"2023-10-17T12:31:36","date":"2023-10-17T08:31:36","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed with Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia in Phase I Clinical Trial (COVALENT-103)","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8226/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-17T12:31:37","lastUpdatedUTC":"2023-10-17T12:31:37"},{"id":8216,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8216","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-2"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for BMF-219 in Type 1 Diabetes","type":{"title":"General","id":3886},"teaser":"BMF-219 is a novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure type 1 diabetes The FDA has cleared the initiation of COVALENT-112, a Phase II clinical trial of BMF-219 in adults with type 1 diabetes (T1D).","language":"en","releaseDate":{"dateUTC":"2023-10-05T12:30:41","date":"2023-10-05T08:30:41","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for BMF-219 in Type 1 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8216/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-10-05T12:30:46","lastUpdatedUTC":"2023-10-05T12:30:46"},{"id":8196,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8196","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-and-health-canada-clearance"},"title":"Biomea Fusion Announces FDA and Health Canada Clearance of the Expansion Cohorts of the Ongoing COVALENT-111 Phase II Study","type":{"title":"General","id":3886},"teaser":"The FDA and Health Canada have cleared the initiation of the expansion portion of COVALENT-111, which will evaluate BMF-219 administered at 100 mg and 200 mg, with dosing durations up to 12 weeks in type 2 diabetes patients The expansion portion will consist of approximately 300 patients and will","language":"en","releaseDate":{"dateUTC":"2023-09-28T12:30:30","date":"2023-09-28T08:30:30","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA and Health Canada Clearance of the Expansion Cohorts of the Ongoing COVALENT-111 Phase II Study","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8196/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-09-28T12:30:34","lastUpdatedUTC":"2023-09-28T12:30:34"},{"id":8171,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8171","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-appointment-juan-pablo-frias-md-chief"},"title":"Biomea Fusion Announces Appointment of Juan Pablo Frías, M.D. as Chief Medical Officer","type":{"title":"General","id":3886},"teaser":"Industry veteran and prominent diabetes clinical development expert to oversee Biomea’s progressing clinical development of novel covalent menin inhibitor BMF-219 in type 2 and type 1 diabetes Steve Morris, M.D., will transition to the role of Chief Development Officer, continuing to lead clinical","language":"en","releaseDate":{"dateUTC":"2023-08-31T12:31:09","date":"2023-08-31T08:31:09","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Appointment of Juan Pablo Frías, M.D. as Chief Medical Officer","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8171/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-08-31T12:31:11","lastUpdatedUTC":"2023-08-31T12:31:11"},{"id":8141,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8141","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported additional positive clinical data at ADA 83 rd Scientific Sessions from the first two cohorts of patients with type 2 diabetes from ongoing Phase I/II study (COVALENT-111) evaluating BMF-219 as a novel, potentially disease-modifying treatment candidate for patients with type 2 diabetes New","language":"en","releaseDate":{"dateUTC":"2023-07-31T20:01:57","date":"2023-07-31T16:01:57","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8141/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-07-31T20:02:01","lastUpdatedUTC":"2023-07-31T20:02:01"},{"id":8126,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8126","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/bmf-219-induces-complete-responses-target-acute-myeloid-leukemia"},"title":"BMF-219 Induces Complete Responses in Target Acute Myeloid Leukemia (AML) Patient Population","type":{"title":"General","id":3886},"teaser":"Initial topline data from COVALENT-101 trial revealed 2 complete responses (CRs) out of 5 relapsed/refractory AML patients carrying menin-dependent mutations treated at Dose Level 4 Dose Level 4 exposure correlates with initial activity seen in BMF-219’s pre-clinical studies Safety profile of","language":"en","releaseDate":{"dateUTC":"2023-07-24T13:00:45","date":"2023-07-24T09:00:45","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"BMF-219 Induces Complete Responses in Target Acute Myeloid Leukemia (AML) Patient Population","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8126/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-07-24T13:00:52","lastUpdatedUTC":"2023-07-24T13:00:52"},{"id":8076,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8076","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-positive-clinical-data-initial-cohorts"},"title":"Biomea Fusion Presents Positive Clinical Data from the Initial Cohorts of the Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes Mellitus at the American Diabetes Association (ADA) 83rd Scientific Sessions; 100 mg Cohort 3 Demonstrated a 90% Response Rate and 70% Maintained or Improved Time in (Normal Glucose) Range, While Off-Treatment","type":{"title":"General","id":3886},"teaser":"Eight weeks after completing treatment with BMF-219, patients with type 2 diabetes (T2D) showed an increase of C-peptide and an improvement of HOMA-B, measured during oral glucose tolerance testing (OGTT), supporting improved beta cell function for these patients Observations of durable and","language":"en","releaseDate":{"dateUTC":"2023-06-24T01:55:00","date":"2023-06-23T21:55:00","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Positive Clinical Data from the Initial Cohorts of the Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes Mellitus at the American Diabetes Association (ADA) 83rd Scientific Sessions; 100 mg Cohort 3 Demonstrated a 90% Response Rate and 70% Maintained or Improved Time in (Normal Glucose) Range, While Off-Treatment","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8076/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-06-24T01:55:04","lastUpdatedUTC":"2023-06-24T02:30:18"},{"id":8066,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8066","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-late-breaking-data-ongoing-phase-ii-0"},"title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes at ADA 2023","type":{"title":"General","id":3886},"teaser":"New clinical data from COVALENT-111 will be unveiled during a late-breaking poster presentation at ADA’s Scientific Sessions BMF-219, an orally delivered novel covalent menin inhibitor, is designed to regenerate, preserve, and reactivate healthy, insulin-producing beta cells Biomea to hold","language":"en","releaseDate":{"dateUTC":"2023-06-20T20:30:48","date":"2023-06-20T16:30:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes at ADA 2023","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/8066/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-06-20T20:30:54","lastUpdatedUTC":"2023-06-20T20:30:54"},{"id":7976,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7976","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2023-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2023 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Reported initial positive clinical data from first two cohorts of Phase II of ongoing Phase I/II study (COVALENT-111) of BMF-219, Biomea’s lead investigational, orally administered covalent menin inhibitor, as a novel, potentially disease-modifying treatment for patients with type 2 diabetes","language":"en","releaseDate":{"dateUTC":"2023-05-02T20:01:50","date":"2023-05-02T16:01:50","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2023 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7976/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-05-02T20:01:57","lastUpdatedUTC":"2023-05-02T20:01:57"},{"id":7961,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7961","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-1"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","type":{"title":"General","id":3886},"teaser":"BMF-500, a novel 3 rd generation covalent inhibitor of fms-like tyrosine kinase 3 (FLT3), is the second investigational compound, discovered and developed by Biomea’s FUSION™ System, to advance to the clinic. Phase I study (COVALENT-103) of BMF-500 will examine its safety and efficacy in patients","language":"en","releaseDate":{"dateUTC":"2023-05-01T12:31:22","date":"2023-05-01T08:31:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory Acute Leukemia","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7961/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-05-01T12:31:33","lastUpdatedUTC":"2023-05-01T12:31:33"},{"id":7921,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7921","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-late-breaking-data-ongoing-phase-ii-trial"},"title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes, at the ADA 83rd Scientific Sessions 2023 in June","type":{"title":"General","id":3886},"teaser":"New clinical data from COVALENT-111 will be featured during the Late-Breaking Poster Presentations at the Scientific Sessions Biomea to hold an investor and KOL event during the Scientific Sessions REDWOOD CITY, Calif., April 19, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2023-04-19T12:30:54","date":"2023-04-19T08:30:54","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Late Breaking Data from Ongoing Phase II Trial, COVALENT-111, Evaluating BMF-219 in Patients with Type 2 Diabetes, at the ADA 83rd Scientific Sessions 2023 in June","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7921/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-19T12:31:00","lastUpdatedUTC":"2023-04-19T12:31:00"},{"id":7906,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7906","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-two-preclinical-posters-114th-aacr-annual"},"title":"Biomea Fusion To Present Two Preclinical Posters at the 114th AACR Annual Meeting","type":{"title":"General","id":3886},"teaser":"Abstract 473 highlights the dose dependent reduction of menin target genes in ex vivo chronic lymphocytic leukemia (CLL) patient samples treated with BMF-219, an investigational covalent inhibitor. BMF-219 showed greater potency and ability to achieve >98% growth inhibition in these CLL patient","language":"en","releaseDate":{"dateUTC":"2023-04-13T20:30:31","date":"2023-04-13T16:30:31","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion To Present Two Preclinical Posters at the 114th AACR Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7906/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-13T20:30:35","lastUpdatedUTC":"2023-04-13T20:30:35"},{"id":7896,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7896","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-closing-upsized-public-offering-and-full"},"title":"Biomea Fusion Announces Closing of Upsized Public Offering and Full Exercise of the Underwriters’ Option to Purchase Additional Shares","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., April 13, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or the “Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically","language":"en","releaseDate":{"dateUTC":"2023-04-13T13:01:10","date":"2023-04-13T09:01:10","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Closing of Upsized Public Offering and Full Exercise of the Underwriters’ Option to Purchase Additional Shares","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7896/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-04-13T13:01:19","lastUpdatedUTC":"2023-04-13T13:01:19"},{"id":7841,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7841","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-pricing-upsized-public-offering-common"},"title":"Biomea Fusion Announces Pricing of Upsized Public Offering of Common Stock","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 29, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-30T03:15:46","date":"2023-03-29T23:15:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Pricing of Upsized Public Offering of Common Stock","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7841/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-30T03:15:55","lastUpdatedUTC":"2023-03-30T03:15:55"},{"id":7831,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7831","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-proposed-public-offering-common-stock"},"title":"Biomea Fusion Announces Proposed Public Offering of Common Stock","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 29, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-29T10:30:50","date":"2023-03-29T06:30:50","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Proposed Public Offering of Common Stock","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7831/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-29T10:30:58","lastUpdatedUTC":"2023-03-29T10:30:58"},{"id":7806,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7806","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-fourth-quarter-and-full-year-2022"},"title":"Biomea Fusion Reports Fourth Quarter and Full Year 2022 Financial Results and Corporate Highlights","type":{"title":"General","id":3886},"teaser":"Expanded clinical development footprint of BMF-219, the company’s lead investigational, orally administered, covalent menin inhibitor, to eight liquid and solid tumor indications and type 2 diabetes across three ongoing clinical trials COVALENT-101 (Phase I study) enrolling four liquid tumor","language":"en","releaseDate":{"dateUTC":"2023-03-28T20:02:28","date":"2023-03-28T16:02:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Fourth Quarter and Full Year 2022 Financial Results and Corporate Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7806/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-28T20:02:30","lastUpdatedUTC":"2023-03-28T20:02:30"},{"id":7796,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7796","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-positive-data-initial-cohorts-ongoing"},"title":"Biomea Fusion Announces Positive Data from Initial Cohorts of Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes; 100 mg Cohort 3 Demonstrated an 89% Response Rate and 1% Median Reduction in HbA1c at Day 28","type":{"title":"General","id":3886},"teaser":"In Cohort 3, after 4 weeks of once-daily 100 mg dosing with the investigational, oral covalent menin inhibitor, BMF-219, 89% of patients achieved a reduction in A1c, 78% of patients achieved at least a 0.5% reduction in A1c, and 56% achieved at least a 1% reduction in A1c.","language":"en","releaseDate":{"dateUTC":"2023-03-28T12:01:22","date":"2023-03-28T08:01:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Positive Data from Initial Cohorts of Ongoing Phase II Study (COVALENT-111) of BMF-219 in Patients with Type 2 Diabetes; 100 mg Cohort 3 Demonstrated an 89% Response Rate and 1% Median Reduction in HbA1c at Day 28","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7796/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-28T12:01:31","lastUpdatedUTC":"2023-03-28T12:01:31"},{"id":7781,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7781","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-host-conference-call-and-webcast-discuss-initial"},"title":"Biomea Fusion to Host Conference Call and Webcast to Discuss Initial Phase II Clinical Data for BMF-219 in Subjects with Type 2 Diabetes on March 28th, 2023 at 8:30 a.m. ET","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 23, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2023-03-23T12:01:38","date":"2023-03-23T08:01:38","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Host Conference Call and Webcast to Discuss Initial Phase II Clinical Data for BMF-219 in Subjects with Type 2 Diabetes on March 28th, 2023 at 8:30 a.m. ET","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7781/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-23T12:01:50","lastUpdatedUTC":"2023-03-27T17:59:34"},{"id":7741,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7741","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-upcoming-investor-events"},"title":"Biomea Fusion To Participate In Upcoming Investor Events","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., March 02, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a biopharmaceutical company focused on the discovery and development of covalent small molecules to treat patients with genetically defined cancers and metabolic diseases, today announced that","language":"en","releaseDate":{"dateUTC":"2023-03-02T13:31:37","date":"2023-03-02T08:31:37","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion To Participate In Upcoming Investor Events","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7741/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-03-02T13:31:40","lastUpdatedUTC":"2023-03-02T13:31:40"},{"id":7686,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7686","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-doses-first-patient-phase-iib-clinical-trial"},"title":"Biomea Fusion Doses First Patient in Phase I/Ib Clinical Trial (COVALENT-102) of BMF-219 in KRAS Mutant Solid Tumors","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first menin inhibitor to be clinically studied in patients with KRAS-mutated non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC) A pan-KRAS inhibitor targeting multiple KRAS mutations (including G12C, G12D and G12R, among others)","language":"en","releaseDate":{"dateUTC":"2023-01-17T13:02:21","date":"2023-01-17T08:02:21","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Doses First Patient in Phase I/Ib Clinical Trial (COVALENT-102) of BMF-219 in KRAS Mutant Solid Tumors","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7686/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-17T13:02:32","lastUpdatedUTC":"2023-01-17T13:02:32"},{"id":7651,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7651","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-41st-annual-jp-morgan-healthcare"},"title":"Biomea Fusion to Present at 41st Annual J.P. Morgan Healthcare Conference and Highlight 2023 Corporate Milestones","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Jan. 09, 2023 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2023-01-09T13:01:27","date":"2023-01-09T08:01:27","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present at 41st Annual J.P. Morgan Healthcare Conference and Highlight 2023 Corporate Milestones","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7651/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-09T13:01:37","lastUpdatedUTC":"2023-01-09T13:01:37"},{"id":7631,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7631","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-patient-type-2-diabetes"},"title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes in the United States in Ongoing Phase I/II (COVALENT-111) Study of BMF-219","type":{"title":"General","id":3886},"teaser":"COVALENT-111, now underway in the US, has completed Phase I and is currently enrolling type 2 diabetes patients in the Phase II randomized, placebo-controlled portion of the trial BMF-219, an orally available covalent menin inhibitor, is being evaluated for its potential to enable the","language":"en","releaseDate":{"dateUTC":"2023-01-04T14:01:17","date":"2023-01-04T09:01:17","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes in the United States in Ongoing Phase I/II (COVALENT-111) Study of BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7631/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2023-01-04T14:01:28","lastUpdatedUTC":"2023-01-04T14:01:28"},{"id":7601,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7601","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-fda-clearance-investigational-new-drug-0"},"title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent Menin Inhibitor BMF-219 in Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"COVALENT-111, a Phase I/II clinical trial in patients with type 2 diabetes, already underway in Canada, will now activate sites in the US As previously reported, the Phase I portion of COVALENT-111 has been completed, with BMF-219 demonstrating a favorable safety, pharmacokinetics (PK) and","language":"en","releaseDate":{"dateUTC":"2022-12-14T21:30:31","date":"2022-12-14T16:30:31","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces FDA Clearance of Investigational New Drug (IND) Application for Covalent Menin Inhibitor BMF-219 in Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7601/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-12-14T21:30:40","lastUpdatedUTC":"2022-12-14T22:24:20"},{"id":7581,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7581","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-2022-ash-annual-meeting-preclinical-data"},"title":"Biomea Fusion Presents at the 2022 ASH Annual Meeting Preclinical Data on BMF-500 Supporting its Potential as the Most Potent and Selective FLT3 Inhibitor to Date","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third generation covalent FLT3 inhibitor, demonstrated preclinically: Picomolar affinity to activating FLT3 mutations including FLT3-ITD and various tyrosine kinase domain (TKD) mutations Multi-fold higher potency and increased cytotoxicity than commercially available","language":"en","releaseDate":{"dateUTC":"2022-12-12T13:00:14","date":"2022-12-12T08:00:14","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents at the 2022 ASH Annual Meeting Preclinical Data on BMF-500 Supporting its Potential as the Most Potent and Selective FLT3 Inhibitor to Date","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7581/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-12-12T13:00:24","lastUpdatedUTC":"2022-12-12T13:00:24"},{"id":7526,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7526","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-piper-sandler-34th-annual-healthcare"},"title":"Biomea Fusion to Participate in Piper Sandler 34th Annual Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., Nov. 11, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”)(Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and","language":"en","releaseDate":{"dateUTC":"2022-11-11T13:30:32","date":"2022-11-11T08:30:32","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Piper Sandler 34th Annual Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7526/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-11T13:30:41","lastUpdatedUTC":"2022-11-11T13:30:41"},{"id":7511,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7511","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-third-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports Third Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"Continued to establish Biomea Fusion as the next-generation leader in covalent medicines Expanded clinical development footprint of BMF-219, the company’s lead investigational, orally administered, covalent menin inhibitor, in multiple liquid and solid tumor indications, including first in class","language":"en","releaseDate":{"dateUTC":"2022-11-07T21:28:19","date":"2022-11-07T16:28:19","timezone":{"name":"America/New_York","code":"EST"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Third Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7511/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-07T21:28:23","lastUpdatedUTC":"2022-11-07T21:28:23"},{"id":7496,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7496","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-preclinical-data-bmf-500-investigational"},"title":"Biomea Fusion to Present Preclinical Data for BMF-500, an Investigational Oral Covalent FLT3 Inhibitor, at the 64th American Society of Hematology (ASH) Annual Meeting","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third generation covalent FLT3 inhibitor, has demonstrated preclinically that it may be the most potent and selective inhibitor of FLT3 evaluated to date: Greater Cytotoxicity: In acute myeloid leukemia (AML) cell lines, three-hour treatment with BMF-500 followed by","language":"en","releaseDate":{"dateUTC":"2022-11-03T13:20:05","date":"2022-11-03T09:20:05","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present Preclinical Data for BMF-500, an Investigational Oral Covalent FLT3 Inhibitor, at the 64th American Society of Hematology (ASH) Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7496/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-11-03T13:20:11","lastUpdatedUTC":"2022-11-03T13:20:11"},{"id":7481,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7481","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-dosing-first-patient-type-2-diabetes-and"},"title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes and Completion of Phase I Healthy Volunteer Portion of Phase I/II (COVALENT-111) Study of BMF-219","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first menin inhibitor to reach the clinic for type 2 diabetes. The Phase II portion of COVALENT-111 is designed to examine the capacity of BMF-219 to provide long-term glycemic control by restoring the patient’s pool of beta cells Beta cell loss is a critical component of the","language":"en","releaseDate":{"dateUTC":"2022-10-31T12:01:54","date":"2022-10-31T08:01:54","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Dosing of First Patient with Type 2 Diabetes and Completion of Phase I Healthy Volunteer Portion of Phase I/II (COVALENT-111) Study of BMF-219","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7481/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-31T12:02:05","lastUpdatedUTC":"2022-10-31T12:02:05"},{"id":7471,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7471","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-chronic-lymphocytic"},"title":"Biomea Fusion Announces First Patient Dosed with Chronic Lymphocytic Leukemia (CLL) in COVALENT-101 Trial","type":{"title":"General","id":3886},"teaser":"COVALENT-101 now includes patients with relapsed/refractory (R/R) CLL BMF-219 is the first menin inhibitor in the clinic for CLL Preclinical data presented at ASCO 2022 demonstrated the potency of BMF-219, a covalent menin inhibitor, across varying cytogenetic risk profiles and Rai stages,","language":"en","releaseDate":{"dateUTC":"2022-10-27T12:01:07","date":"2022-10-27T08:01:07","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed with Chronic Lymphocytic Leukemia (CLL) in COVALENT-101 Trial","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7471/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-27T12:01:18","lastUpdatedUTC":"2022-10-27T12:01:18"},{"id":7441,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7441","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/bmf-219-enters-clinic-kras-solid-tumors"},"title":"BMF-219 Enters the Clinic for KRAS Solid Tumors","type":{"title":"General","id":3886},"teaser":"Biomea Fusion announces FDA clearance of Investigational New Drug (IND) application for covalent menin inhibitor BMF-219 in KRAS solid tumors. Biomea Fusion will now initiate a Phase I/Ib clinical trial (COVALENT-102) of BMF-219 as a monotherapy in patients who have unresectable, locally advanced,","language":"en","releaseDate":{"dateUTC":"2022-10-14T13:00:34","date":"2022-10-14T09:00:34","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"BMF-219 Enters the Clinic for KRAS Solid Tumors","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7441/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-10-14T13:00:42","lastUpdatedUTC":"2022-10-14T13:00:42"},{"id":7416,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7416","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-new-translational-data-european"},"title":"Biomea Fusion Presents New Translational Data at the European Association for the Study of Diabetes (EASD) 2022 in Animal Models and Ex-vivo Human Donor Islets Further Supporting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Treatment with BMF-219 led to an increase in beta cell mass in ex-vivo experiments with human donor islets BMF-219 showed improved pancreatic beta-cell function and beta cell area, insulin sensitivity, blood lipid levels, weight decline, and glycemic control in the rat models (ZDF (Zucker Diabetic","language":"en","releaseDate":{"dateUTC":"2022-09-22T13:30:32","date":"2022-09-22T09:30:32","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents New Translational Data at the European Association for the Study of Diabetes (EASD) 2022 in Animal Models and Ex-vivo Human Donor Islets Further Supporting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7416/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-09-22T13:30:41","lastUpdatedUTC":"2022-09-22T13:30:41"},{"id":7406,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7406","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-new-preclinical-data-european-association"},"title":"Biomea Fusion Presents New Preclinical Data at the European Association for the Study of Diabetes (EASD) Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Menin, a transcriptional scaffold protein, regulates pancreatic beta cell homeostasis; inhibiting menin function with BMF-219 increased beta cell function in a preclinical animal model, driving an improvement in glycemic control and insulin sensitivity.","language":"en","releaseDate":{"dateUTC":"2022-09-20T11:30:15","date":"2022-09-20T07:30:15","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents New Preclinical Data at the European Association for the Study of Diabetes (EASD) Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7406/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-09-20T11:30:22","lastUpdatedUTC":"2022-09-20T11:30:22"},{"id":7386,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7386","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-additional-preclinical-data-demonstrating"},"title":"Biomea Fusion Presents Additional Preclinical Data Demonstrating Anti-Tumor Activity and Mechanistic Evidence for BMF-219 in Diffuse Large B-Cell Lymphoma and Multiple Myeloma Models at International Myeloma Society Annual Meeting","type":{"title":"General","id":3886},"teaser":"Data demonstrated robust anti-tumor activity of BMF-219 and mechanistic evidence for novel inhibition of menin protein in preclinical models of Diffuse Large B-cell Lymphoma (DLBCL) and multiple myeloma (MM). BMF-219 displayed single agent potency, surpassing greater than 90% inhibition at","language":"en","releaseDate":{"dateUTC":"2022-08-26T12:30:53","date":"2022-08-26T08:30:53","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Additional Preclinical Data Demonstrating Anti-Tumor Activity and Mechanistic Evidence for BMF-219 in Diffuse Large B-Cell Lymphoma and Multiple Myeloma Models at International Myeloma Society Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7386/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-08-26T12:31:31","lastUpdatedUTC":"2022-08-26T12:31:31"},{"id":7346,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7346","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-second-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports Second Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"Continued to make significant progress advancing BMF-219, the company’s lead investigational orally administered covalent menin inhibitor, in multiple oncology indications COVALENT-101 (Phase I) study is enrolling for patients with Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML),","language":"en","releaseDate":{"dateUTC":"2022-08-01T20:05:35","date":"2022-08-01T16:05:35","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Second Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7346/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-08-01T20:05:39","lastUpdatedUTC":"2022-08-01T20:05:39"},{"id":7326,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7326","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-selected-two-oral-presentations-european"},"title":"Biomea Fusion Selected for Two Oral Presentations at the European Association for the Study of Diabetes Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting, Acute Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Biomea to present two oral abstracts across multiple animal models highlighting the ability of BMF-219, a covalent menin inhibitor, to significantly lower HbA1C (approximately two-fold greater reduction than active control, liraglutide) and to increase beta cell function.","language":"en","releaseDate":{"dateUTC":"2022-07-01T12:30:12","date":"2022-07-01T08:30:12","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Selected for Two Oral Presentations at the European Association for the Study of Diabetes Annual Meeting Describing BMF-219’s Potential as a Novel, Oral, Long-Acting, Acute Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7326/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-07-01T12:30:17","lastUpdatedUTC":"2022-07-01T12:30:17"},{"id":7276,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7276","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-first-patient-dosed-multiple-myeloma"},"title":"Biomea Fusion Announces First Patient Dosed in Multiple Myeloma Cohort of COVALENT-101 Trial","type":{"title":"General","id":3886},"teaser":"BMF-219, a covalent menin inhibitor, is the first menin inhibitor administered to patients with relapsed/refractory (R/R) multiple myeloma (MM) Patients with R/R MM and R/R diffuse large B-cell lymphoma (DLBCL) are eligible for enrollment in COVALENT-101 Enrollment continuing for both acute","language":"en","releaseDate":{"dateUTC":"2022-06-22T12:30:34","date":"2022-06-22T08:30:34","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces First Patient Dosed in Multiple Myeloma Cohort of COVALENT-101 Trial","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7276/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-22T12:30:44","lastUpdatedUTC":"2022-06-22T12:30:44"},{"id":7246,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7246","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-presents-novel-preclinical-data-ada-2022"},"title":"Biomea Fusion Presents Novel Preclinical Data at ADA 2022 Suggesting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","type":{"title":"General","id":3886},"teaser":"Menin acts as a ‘brake’ on beta cell regeneration; inhibiting menin function with BMF-219 may increase beta cell production and function, thereby increasing insulin levels and controlling high glucose levels BMF-219, a covalent menin inhibitor, showed strong, prolonged glycemic control, insulin","language":"en","releaseDate":{"dateUTC":"2022-06-04T15:00:48","date":"2022-06-04T11:00:48","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Presents Novel Preclinical Data at ADA 2022 Suggesting BMF-219’s Potential as an Oral, Long-Acting Treatment for Type 2 Diabetes","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7246/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-04T15:00:59","lastUpdatedUTC":"2022-06-04T15:00:59"},{"id":7221,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7221","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-jefferies-healthcare-conference"},"title":"Biomea Fusion to Participate in Jefferies Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., June 02, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-06-02T22:00:08","date":"2022-06-02T18:00:08","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Jefferies Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7221/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-02T22:00:21","lastUpdatedUTC":"2022-06-02T22:00:21"},{"id":7206,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7206","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-preclinical-data-bmf-219-two-diabetic"},"title":"Biomea Fusion to Present New Preclinical Data on BMF-219 in Two Diabetic Animal Models at ADA 2022","type":{"title":"General","id":3886},"teaser":"Company to host virtual investor R&D event on Monday, June 6, 2022 at 4:05 PM EDT featuring covalent menin inhibitor, BMF-219, as a potential novel treatment for type 2 diabetes REDWOOD CITY, Calif., June 01, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc.","language":"en","releaseDate":{"dateUTC":"2022-06-01T12:30:46","date":"2022-06-01T08:30:46","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Preclinical Data on BMF-219 in Two Diabetic Animal Models at ADA 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7206/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-06-01T12:30:50","lastUpdatedUTC":"2022-06-01T12:30:50"},{"id":7196,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7196","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-present-new-preclinical-data-showing-bmf-219s"},"title":"Biomea Fusion to Present New Preclinical Data Showing BMF-219’s Strong Activity in Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) Tumor Models at ASCO 2022","type":{"title":"General","id":3886},"teaser":"BMF-219 is the first investigational menin inhibitor in clinical development to show potential as a therapeutic agent for CLL BMF-219, a covalent menin inhibitor, demonstrated potency across ex vivo CLL tumor models with varying cytogenetic risk profiles and Rai stages, indicating broad activity","language":"en","releaseDate":{"dateUTC":"2022-05-26T22:30:28","date":"2022-05-26T18:30:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Present New Preclinical Data Showing BMF-219’s Strong Activity in Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) Tumor Models at ASCO 2022","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7196/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-26T22:30:31","lastUpdatedUTC":"2022-05-26T22:30:31"},{"id":7181,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7181","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-attend-hc-wainwright-global-investment-conference"},"title":"Biomea Fusion to Attend H.C. Wainwright Global Investment Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., May 20, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-05-20T17:30:31","date":"2022-05-20T13:30:31","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Attend H.C. Wainwright Global Investment Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7181/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-20T17:30:42","lastUpdatedUTC":"2022-05-20T17:30:42"},{"id":7166,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7166","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-ind-candidate-selection-bmf-500"},"title":"Biomea Fusion Announces IND Candidate Selection: BMF-500, a Potential Best-in-Class Oral Covalent Inhibitor of FLT3","type":{"title":"General","id":3886},"teaser":"BMF-500, an investigational third-generation covalent inhibitor of FLT3, demonstrated picomolar IC 50 values across key FLT3 isoforms, potentially making it the most potent inhibitor of its class Highly selective for FLT3, BMF-500 was observed to avoid other type III receptor tyrosine kinase (RTK)","language":"en","releaseDate":{"dateUTC":"2022-05-19T12:00:39","date":"2022-05-19T08:00:39","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces IND Candidate Selection: BMF-500, a Potential Best-in-Class Oral Covalent Inhibitor of FLT3","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7166/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-19T12:00:48","lastUpdatedUTC":"2022-05-19T12:00:48"},{"id":7146,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7146","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-first-quarter-2022-financial-results-and"},"title":"Biomea Fusion Reports First Quarter 2022 Financial Results and Business Highlights","type":{"title":"General","id":3886},"teaser":"COVALENT-101 study continues to enroll relapsed/refractory (R/R) acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL) patients and has included R/R diffuse large B-cell lymphoma (DLBCL) and R/R multiple myeloma (MM) patients to the study.","language":"en","releaseDate":{"dateUTC":"2022-05-16T11:30:22","date":"2022-05-16T07:30:22","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports First Quarter 2022 Financial Results and Business Highlights","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7146/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-16T11:30:30","lastUpdatedUTC":"2022-05-16T11:30:30"},{"id":7131,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7131","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-participate-bank-america-securities-2022"},"title":"Biomea Fusion to Participate in Bank of America Securities 2022 Healthcare Conference","type":{"title":"General","id":3886},"teaser":"REDWOOD CITY, Calif., May 09, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing novel covalent small molecules to treat and improve the lives of patients with genetically defined cancers and metabolic","language":"en","releaseDate":{"dateUTC":"2022-05-09T20:30:13","date":"2022-05-09T16:30:13","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion to Participate in Bank of America Securities 2022 Healthcare Conference","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7131/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-05-09T20:30:25","lastUpdatedUTC":"2022-05-09T20:30:25"},{"id":7106,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7106","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-announces-acceptance-multiple-abstracts-2022-asco"},"title":"Biomea Fusion Announces Acceptance of Multiple Abstracts at the 2022 ASCO Annual Meeting","type":{"title":"General","id":3886},"teaser":"Biomea to present preclinical data in chronic lymphocytic leukemia (CLL) and Trial In Progress (TIP) information for its ongoing COVALENT-101 Phase I trial REDWOOD CITY, Calif., April 27, 2022 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (Nasdaq: BMEA), a clinical-stage biopharmaceutical company","language":"en","releaseDate":{"dateUTC":"2022-04-27T20:00:29","date":"2022-04-27T16:00:29","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Announces Acceptance of Multiple Abstracts at the 2022 ASCO Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7106/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-04-27T20:00:40","lastUpdatedUTC":"2022-04-27T20:00:40"},{"id":7081,"link":{"url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7081","hostedUrl":"https://investors.biomeafusion.com/news-releases/news-release-details/biomea-fusion-reports-preclinical-data-bmf-219-and-trial"},"title":"Biomea Fusion Reports Preclinical Data on BMF-219 and Trial in Progress Presentations at AACR 2022 Annual Meeting","type":{"title":"General","id":3886},"teaser":"Covalent menin inhibitor BMF-219 showed strong cytotoxic activity as a single agent at similar concentrations across multiple preclinical patient derived (PDX) models ex vivo , including diffuse large B-cell lymphoma (DLBCL), multiple myeloma (MM), colorectal cancer (CRC), non-small cell lung","language":"en","releaseDate":{"dateUTC":"2022-04-08T17:00:28","date":"2022-04-08T13:00:28","timezone":{"name":"America/New_York","code":"EDT"}},"body":[{"type":"html","link":{"id":null,"source":"api","title":"Biomea Fusion Reports Preclinical Data on BMF-219 and Trial in Progress Presentations at AACR 2022 Annual Meeting","url":"https://clientapi.gcs-web.com/data/1f6273f2-ca70-4b64-b0d0-9a6176fa6866/news/7081/html"}}],"additionalFormats":[],"categories":[],"unpublishOn":null,"thumbnail":null,"createdOnUTC":"2022-04-08T17:01:20","lastUpdatedUTC":"2022-04-08T17:01:20"}],"error":null};
var latestArticle = prData.data[0];
document.addEventListener("DOMContentLoaded", function() {
// console.log(document.getElementsByClassName('njt-nofi-text'));
document.getElementsByClassName('njt-nofi-text')[0].innerHTML = '
' + latestArticle.title + '';
// console.log(latestArticle);
});
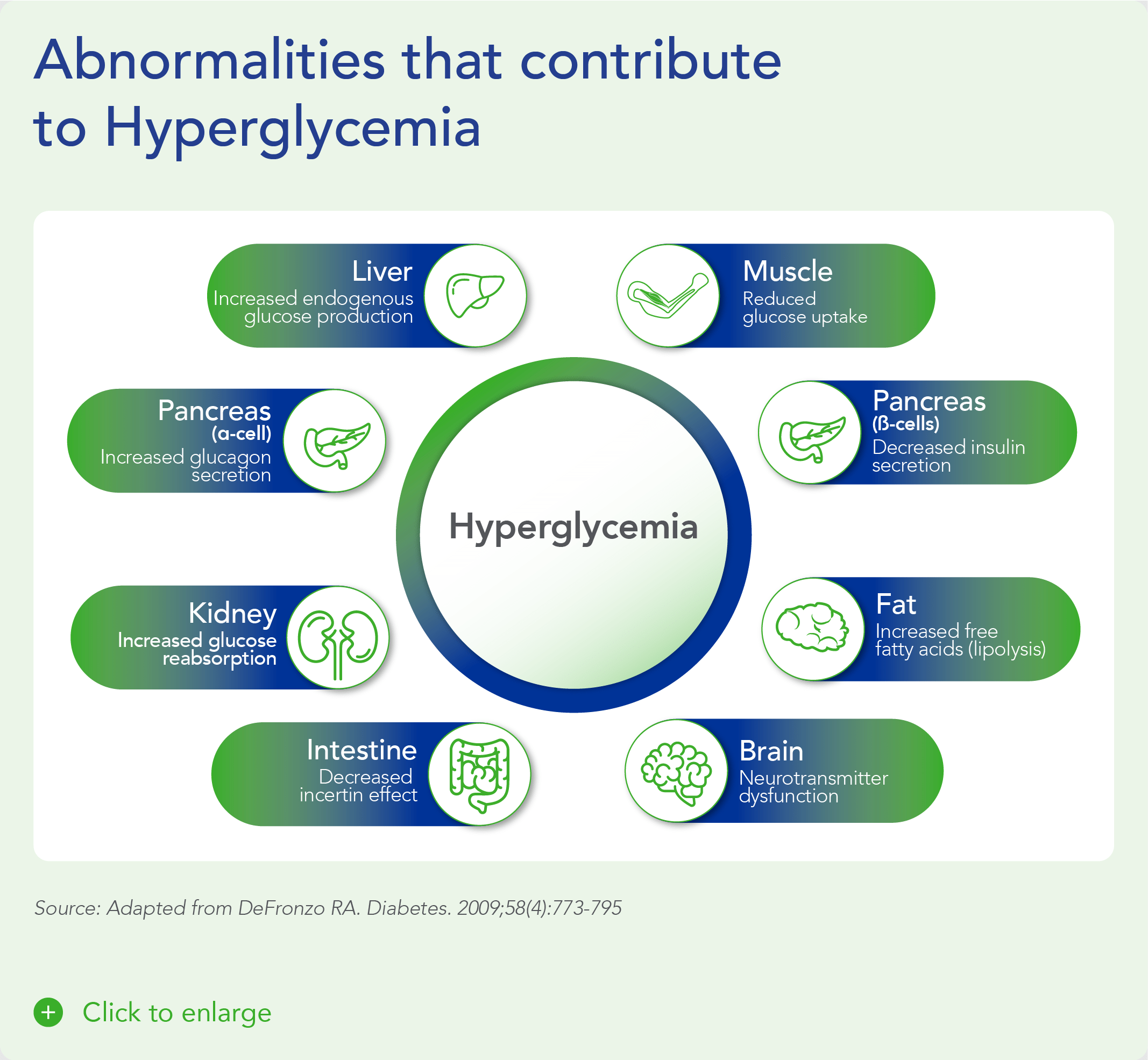
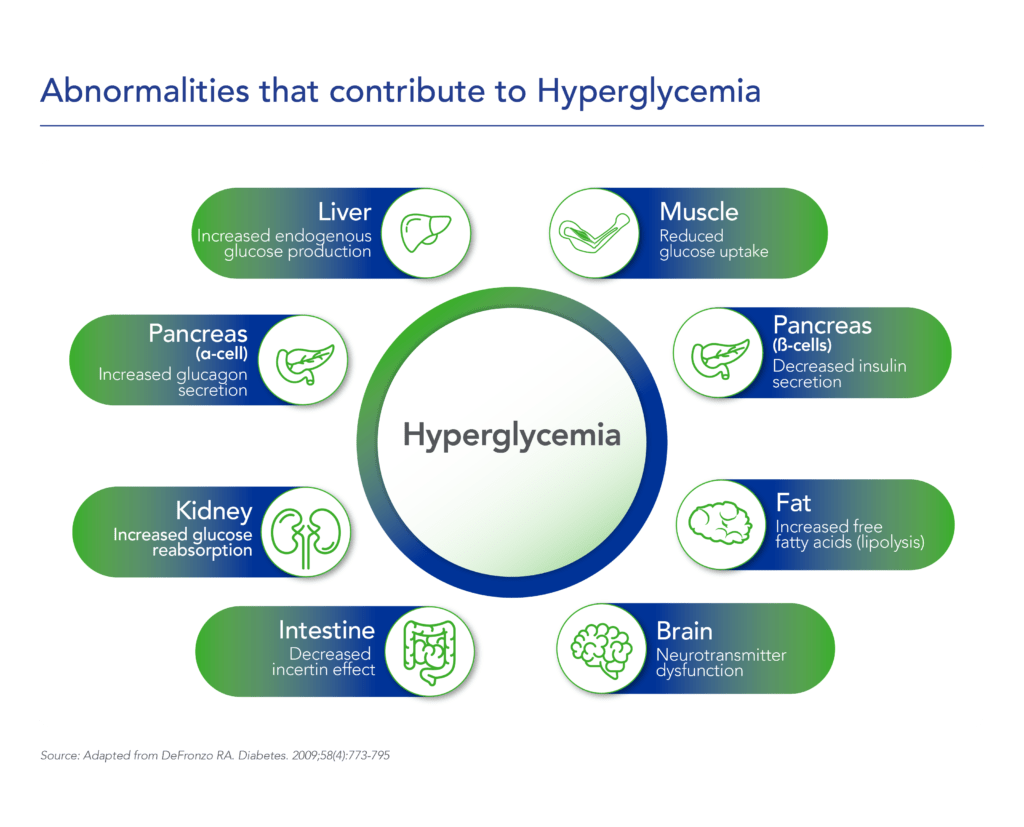
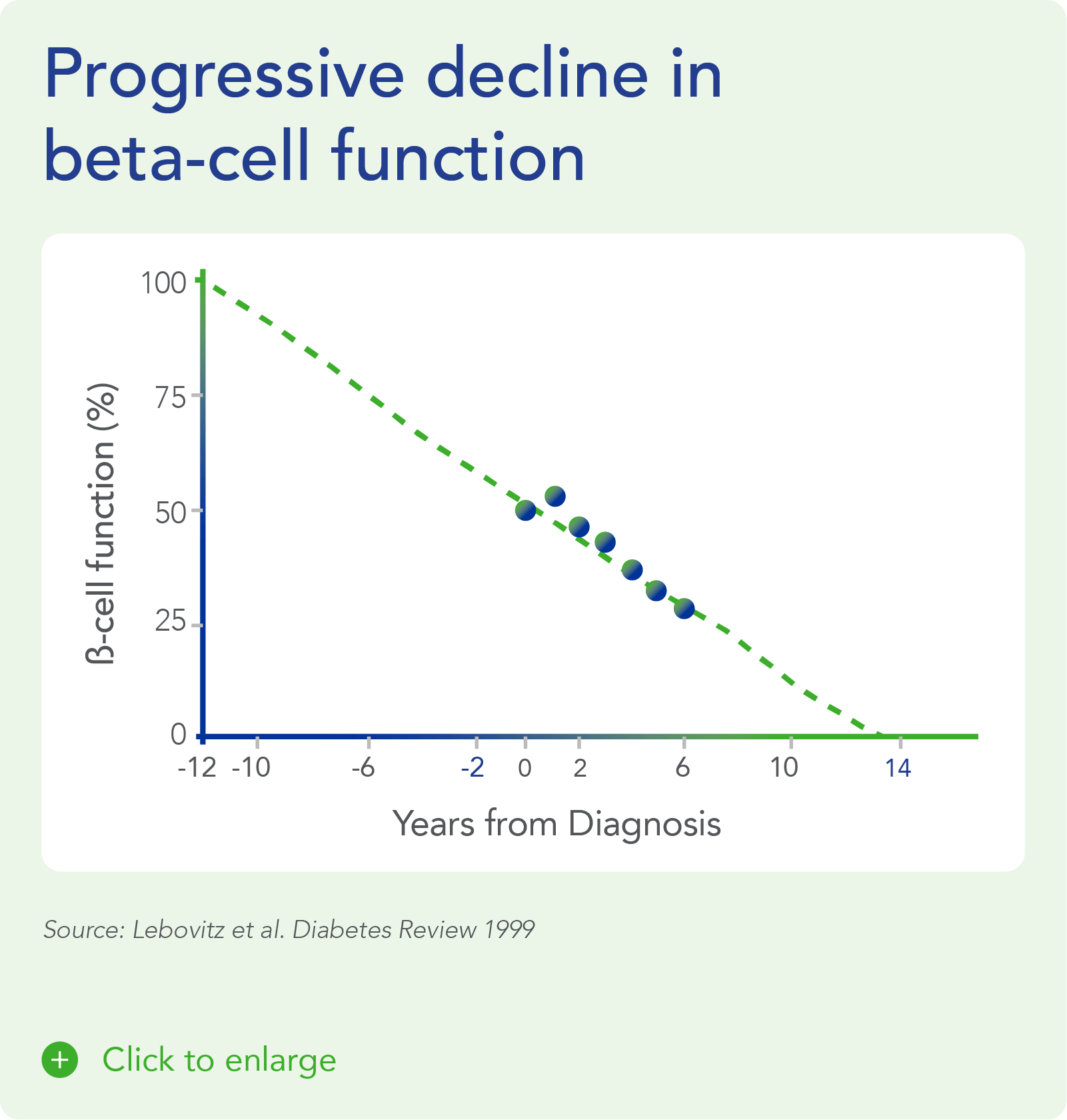

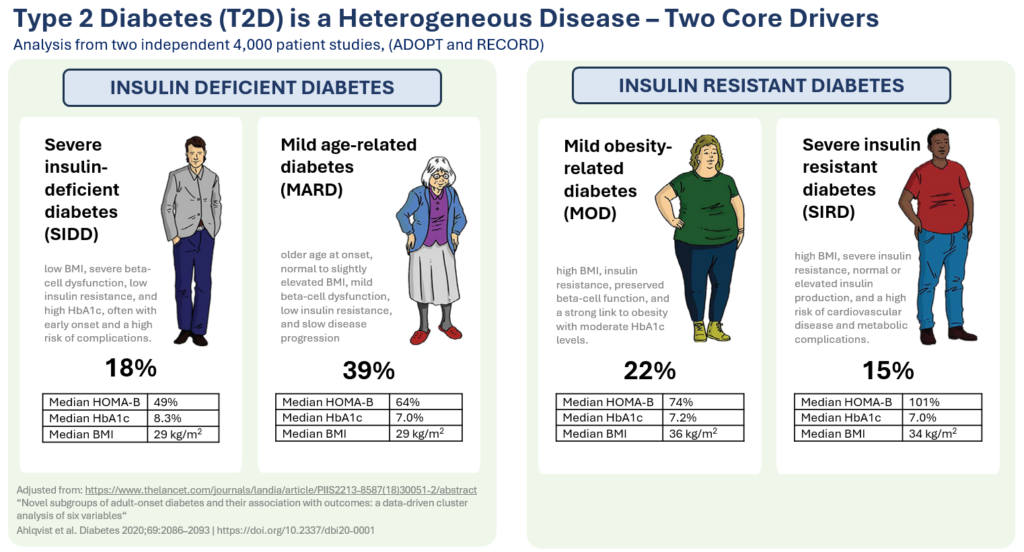

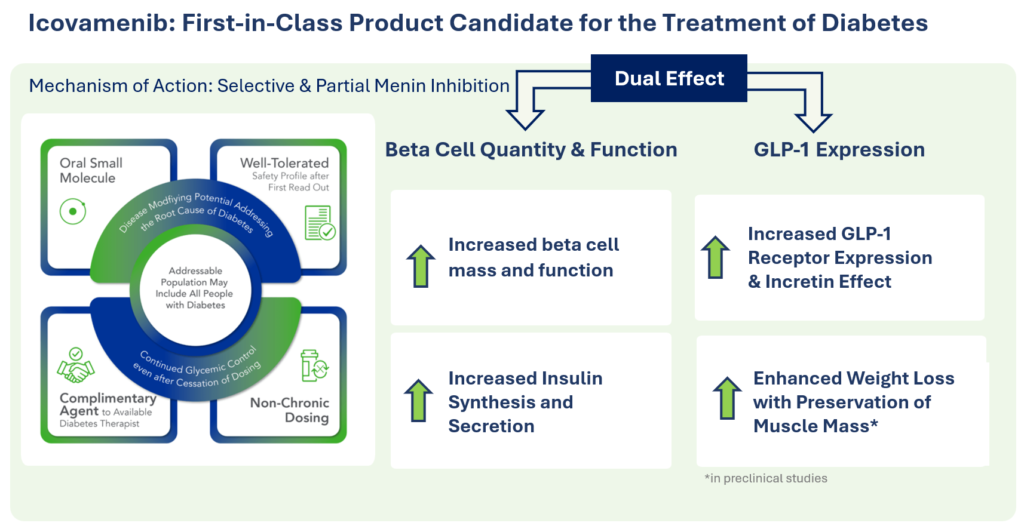


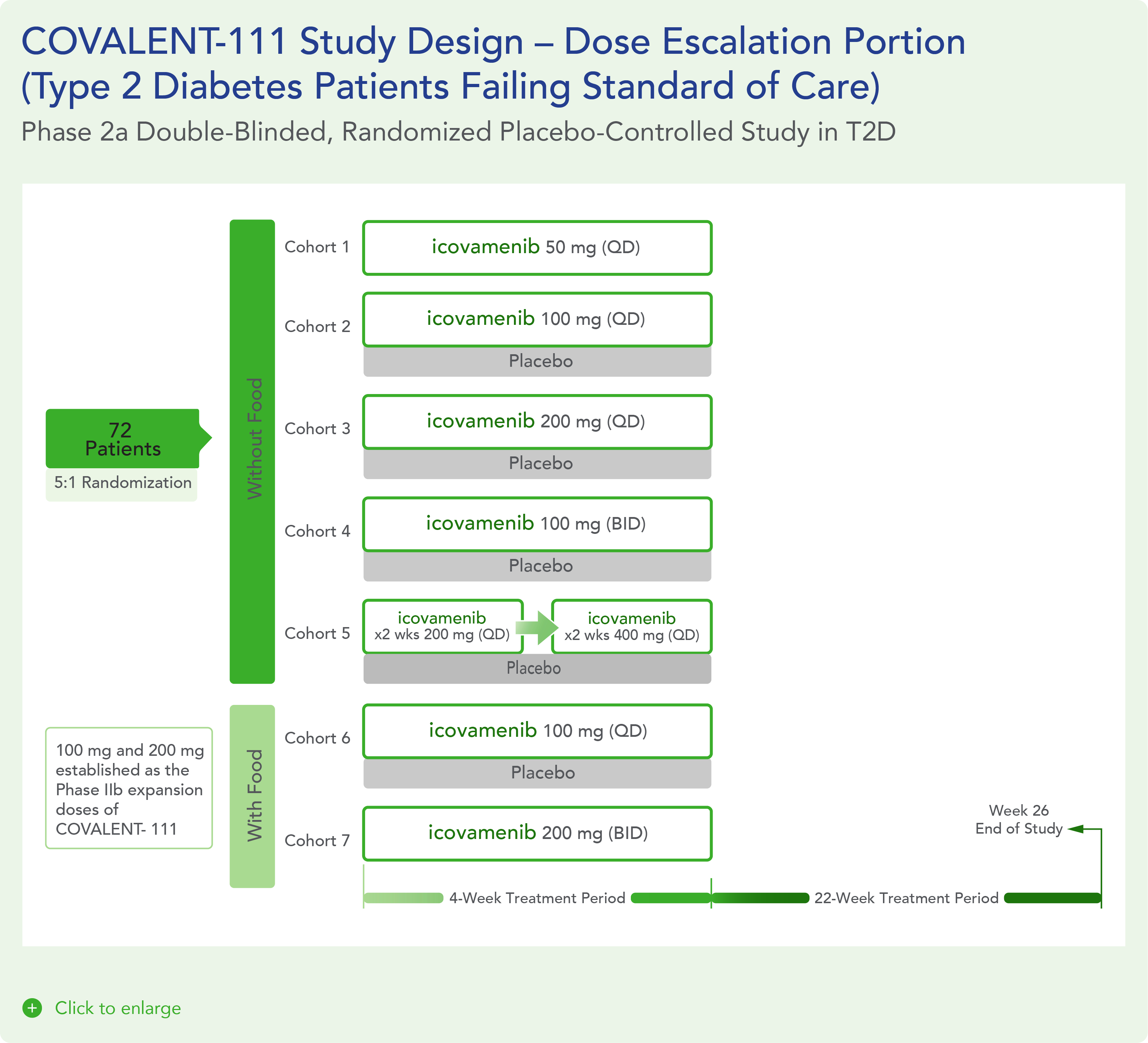
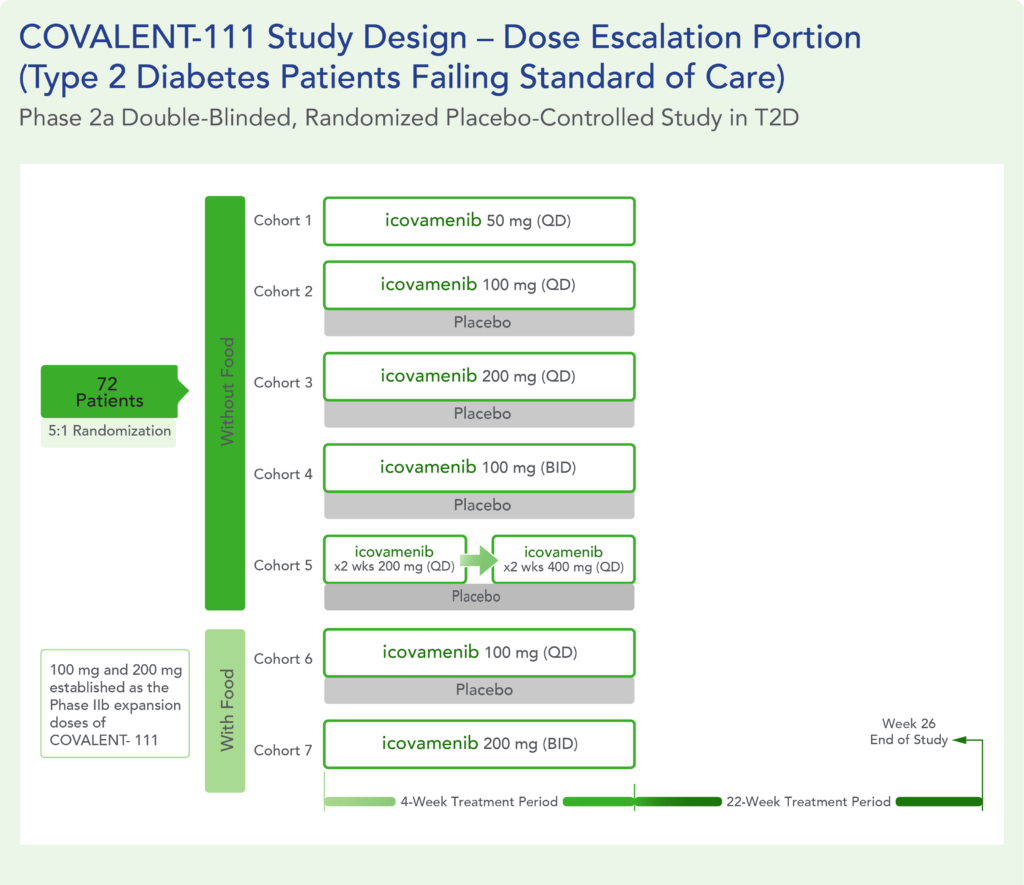
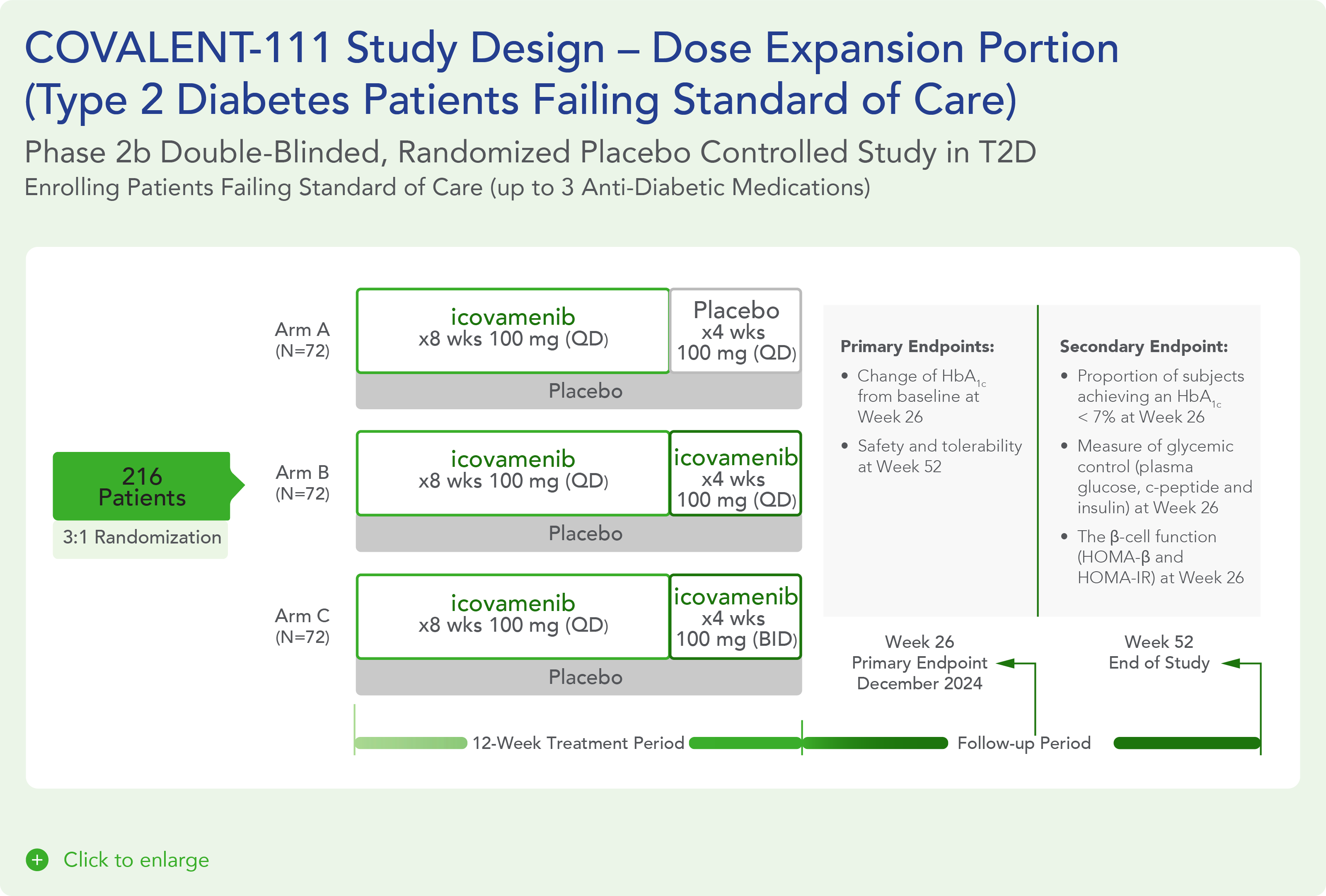
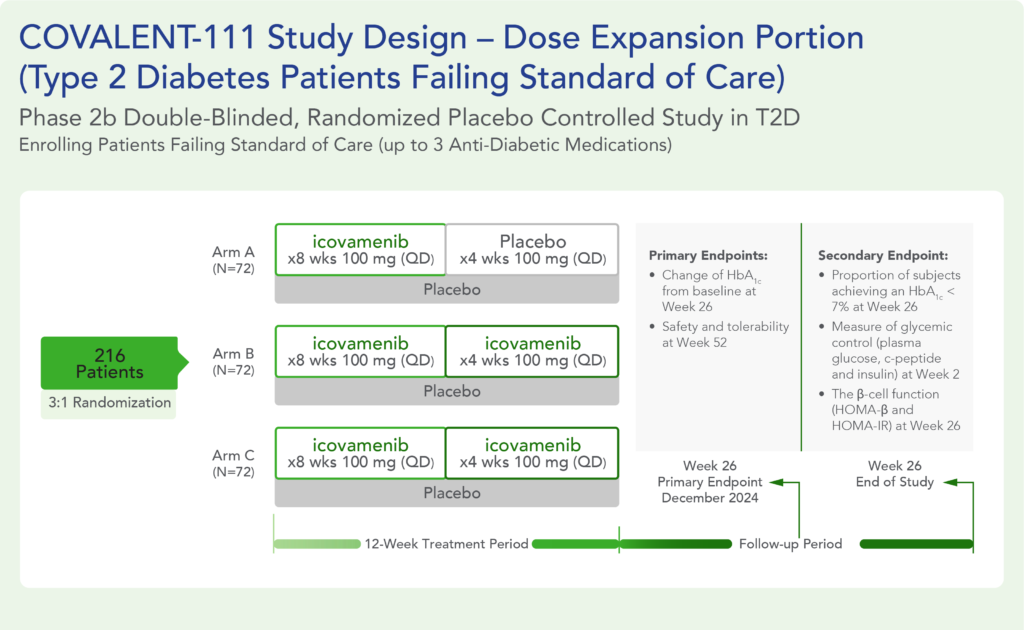
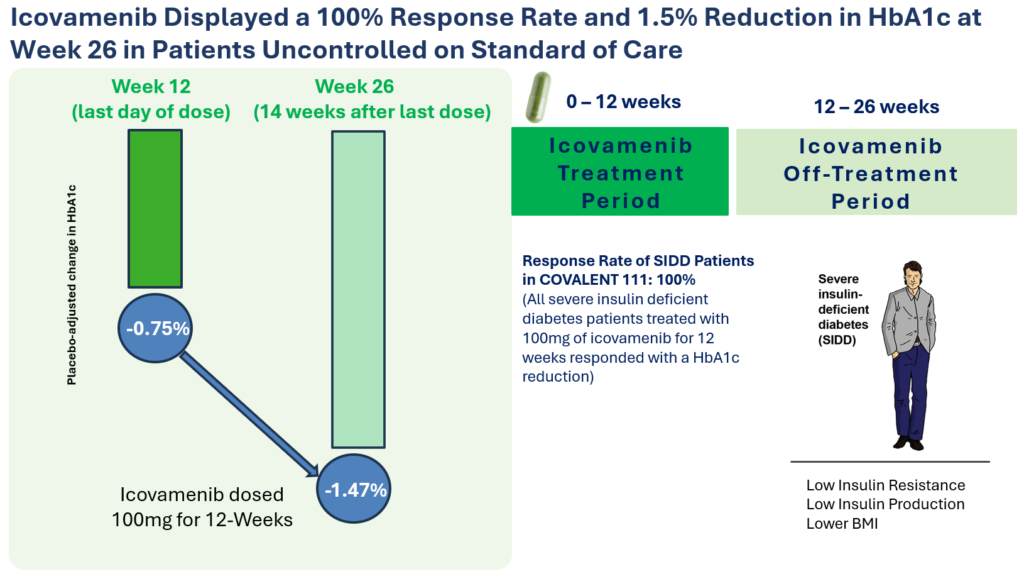
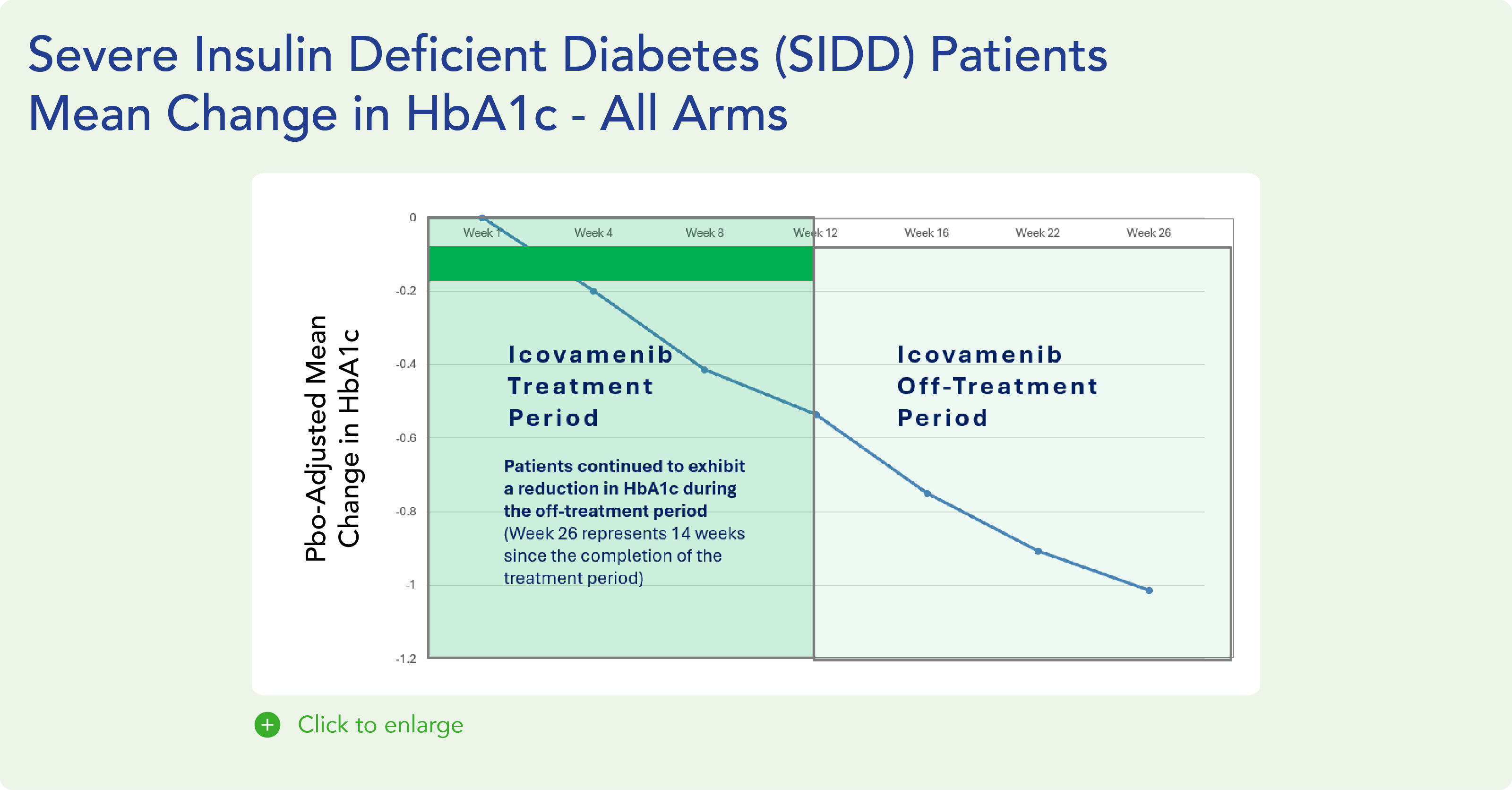
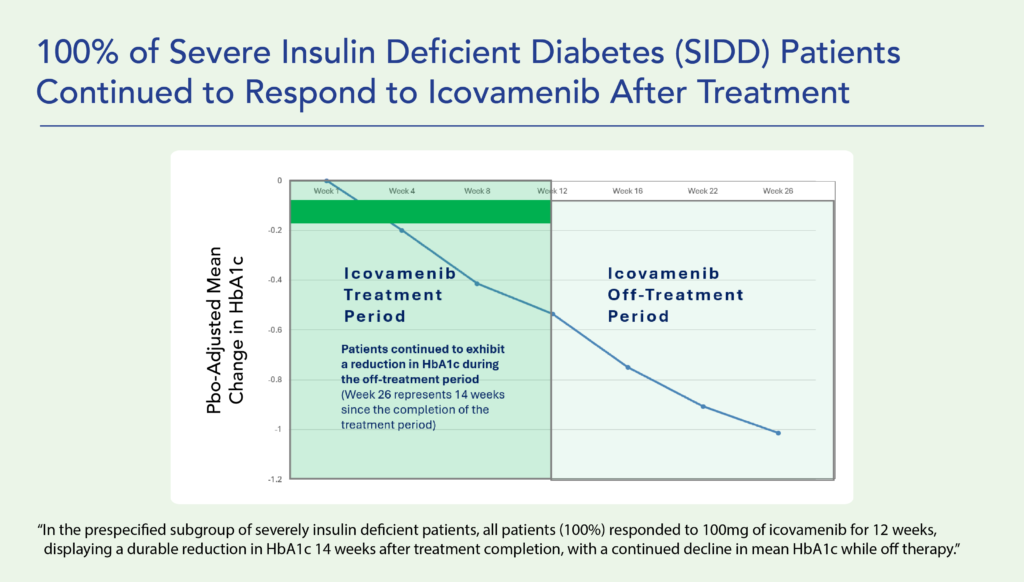
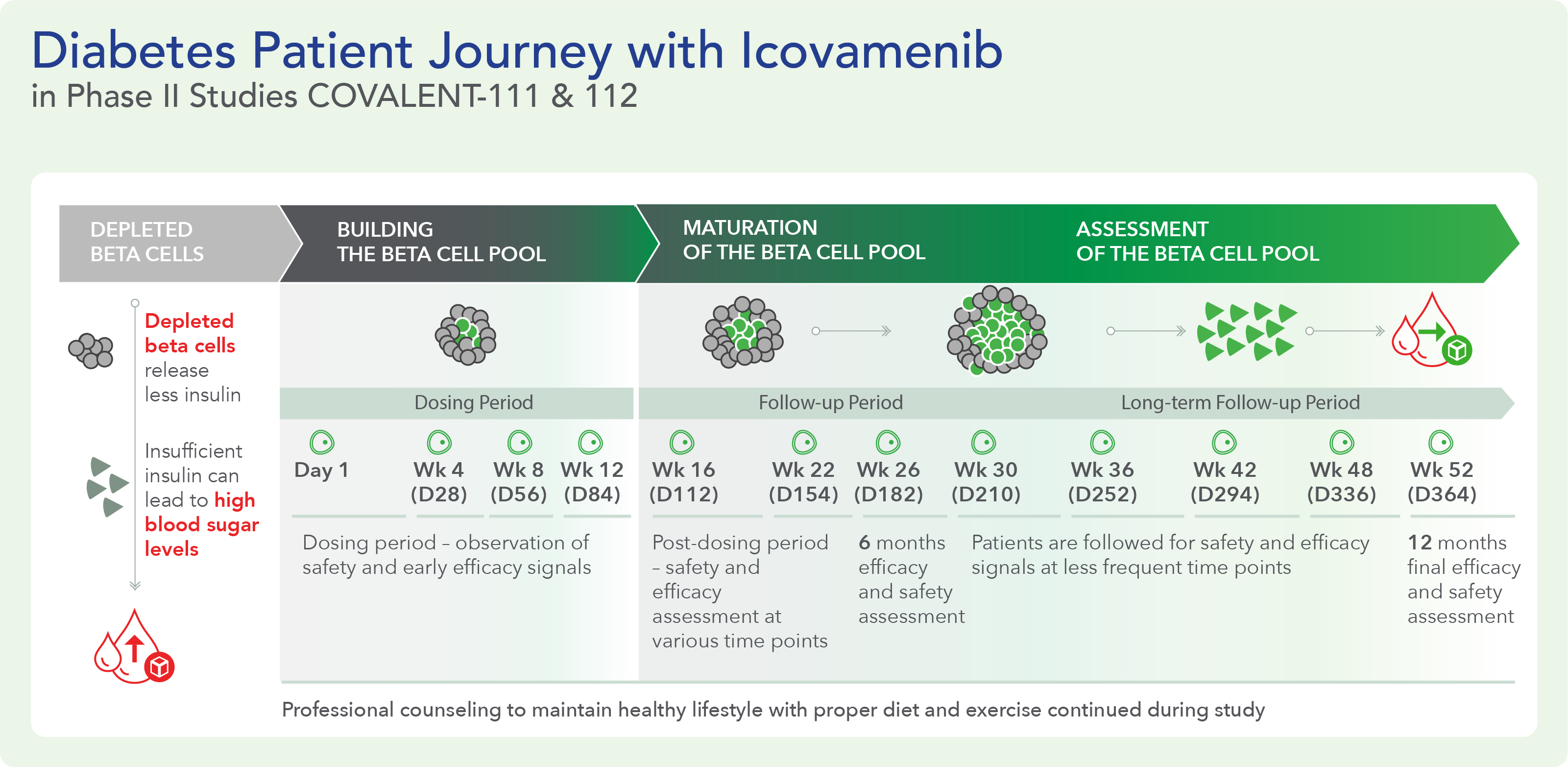
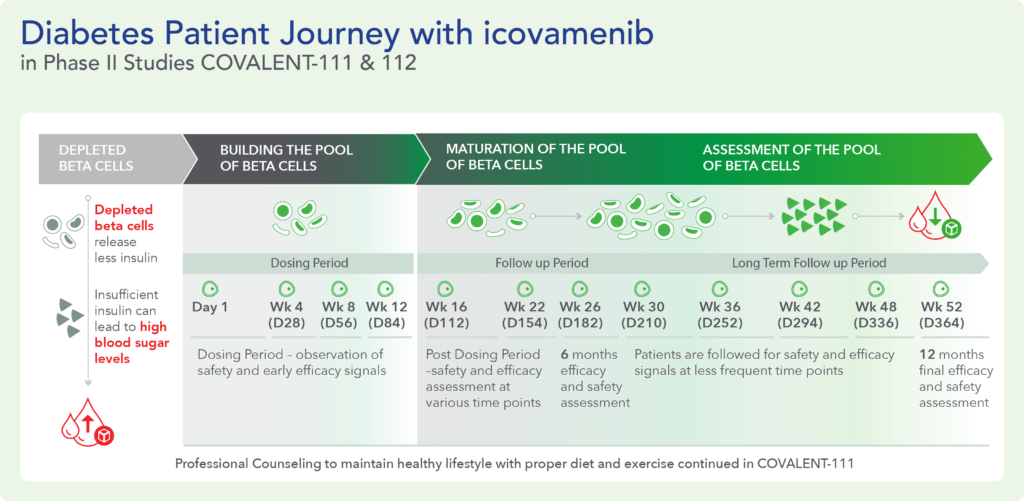
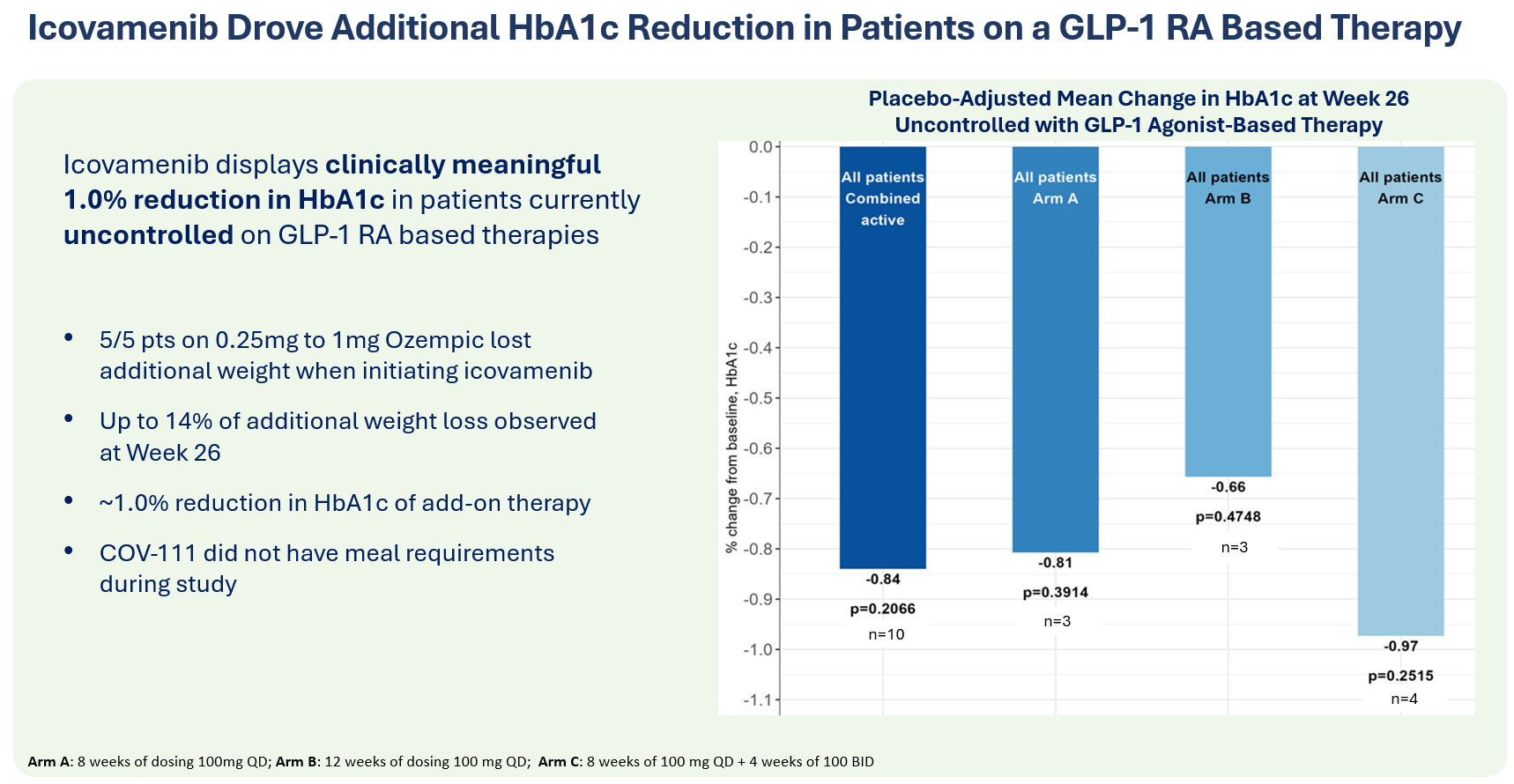
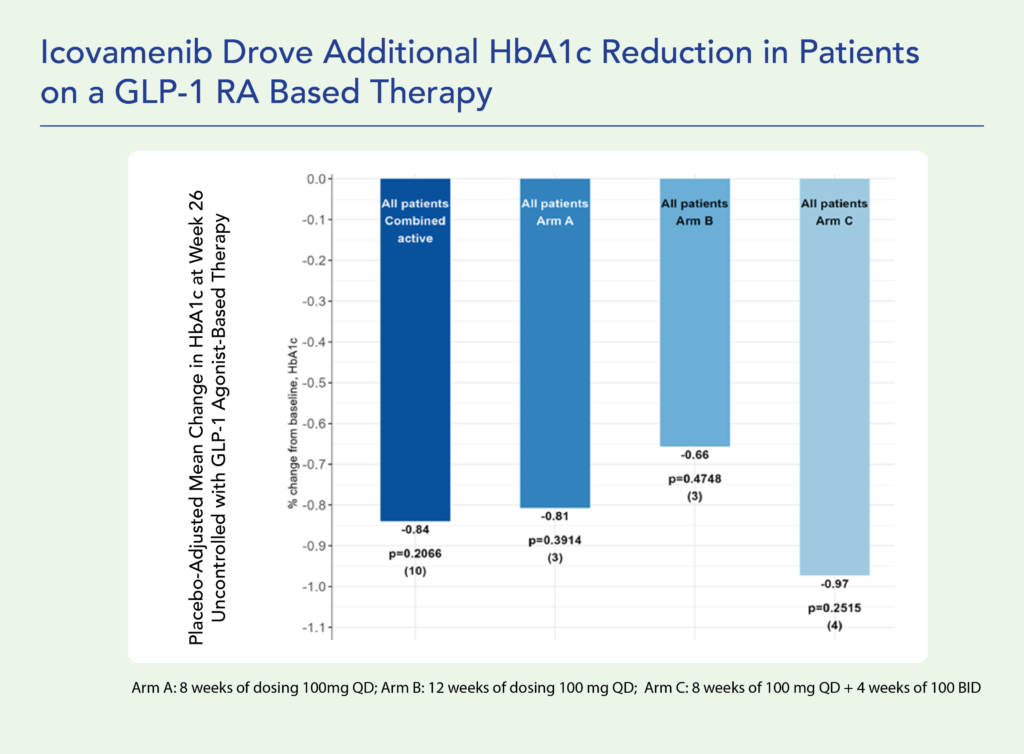
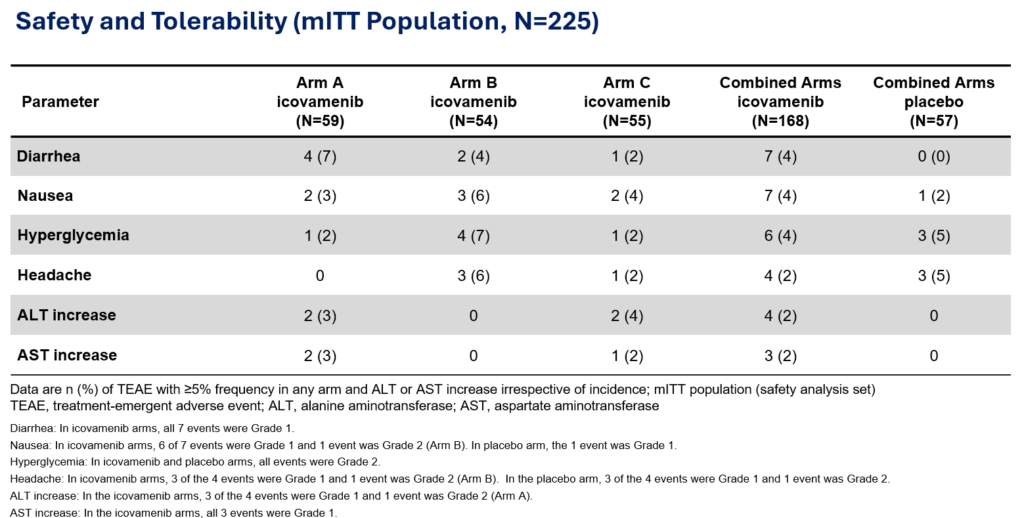
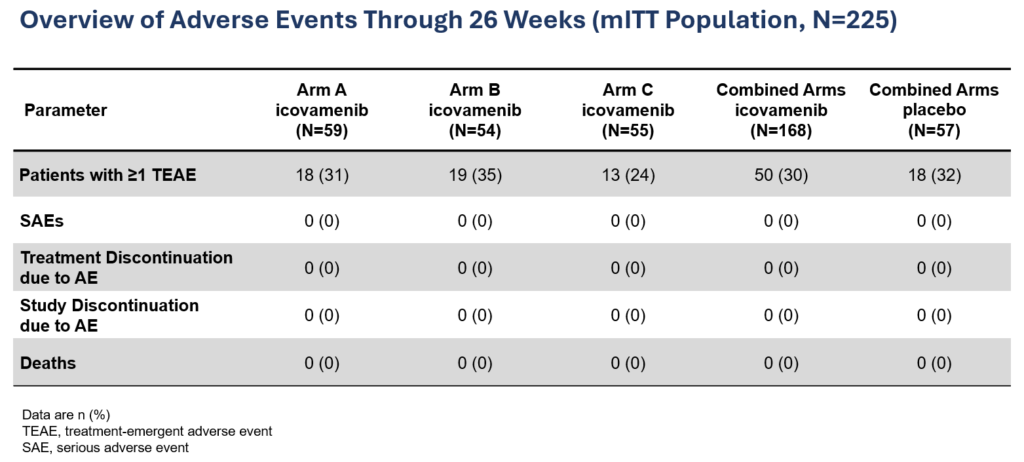
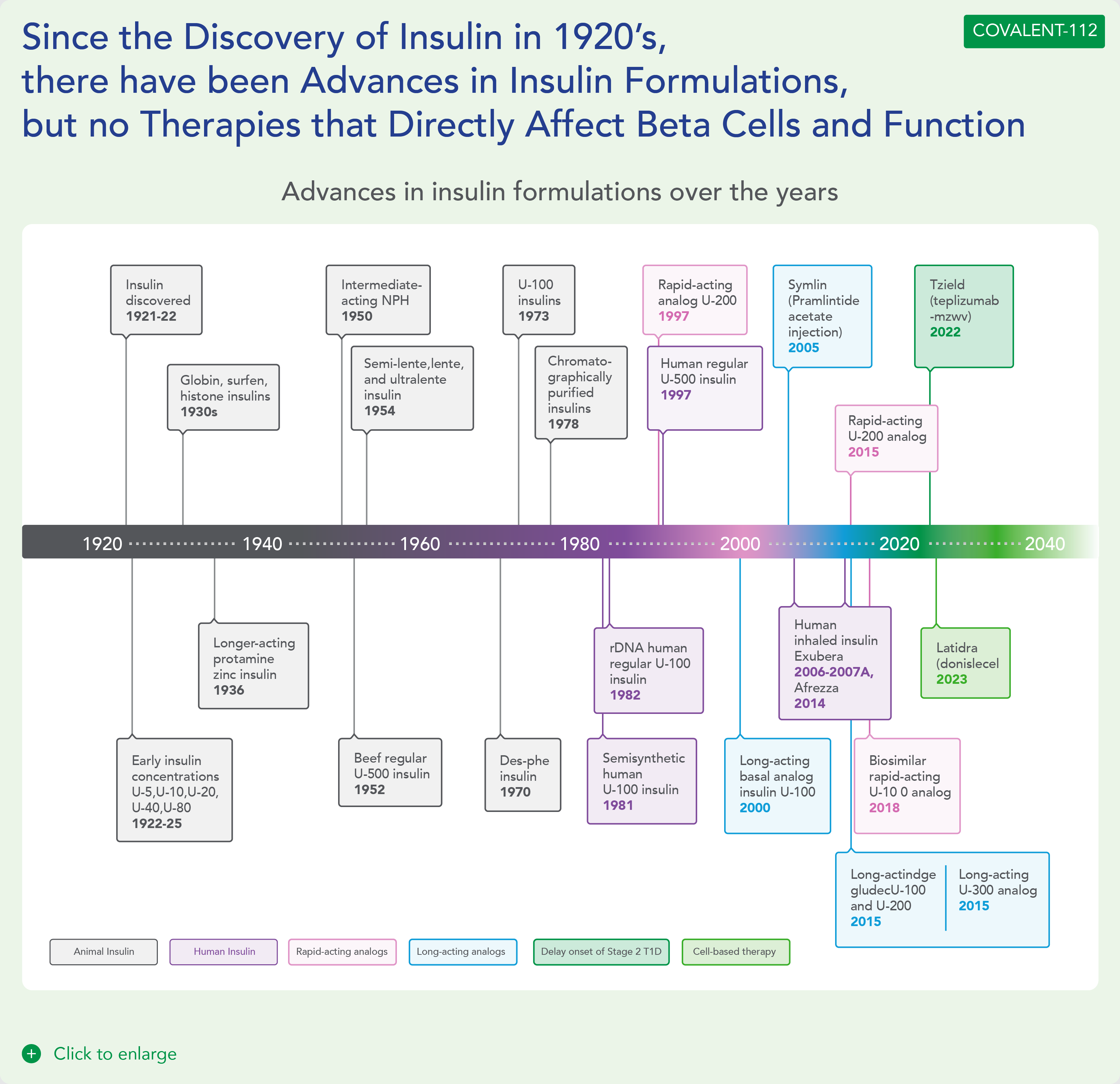

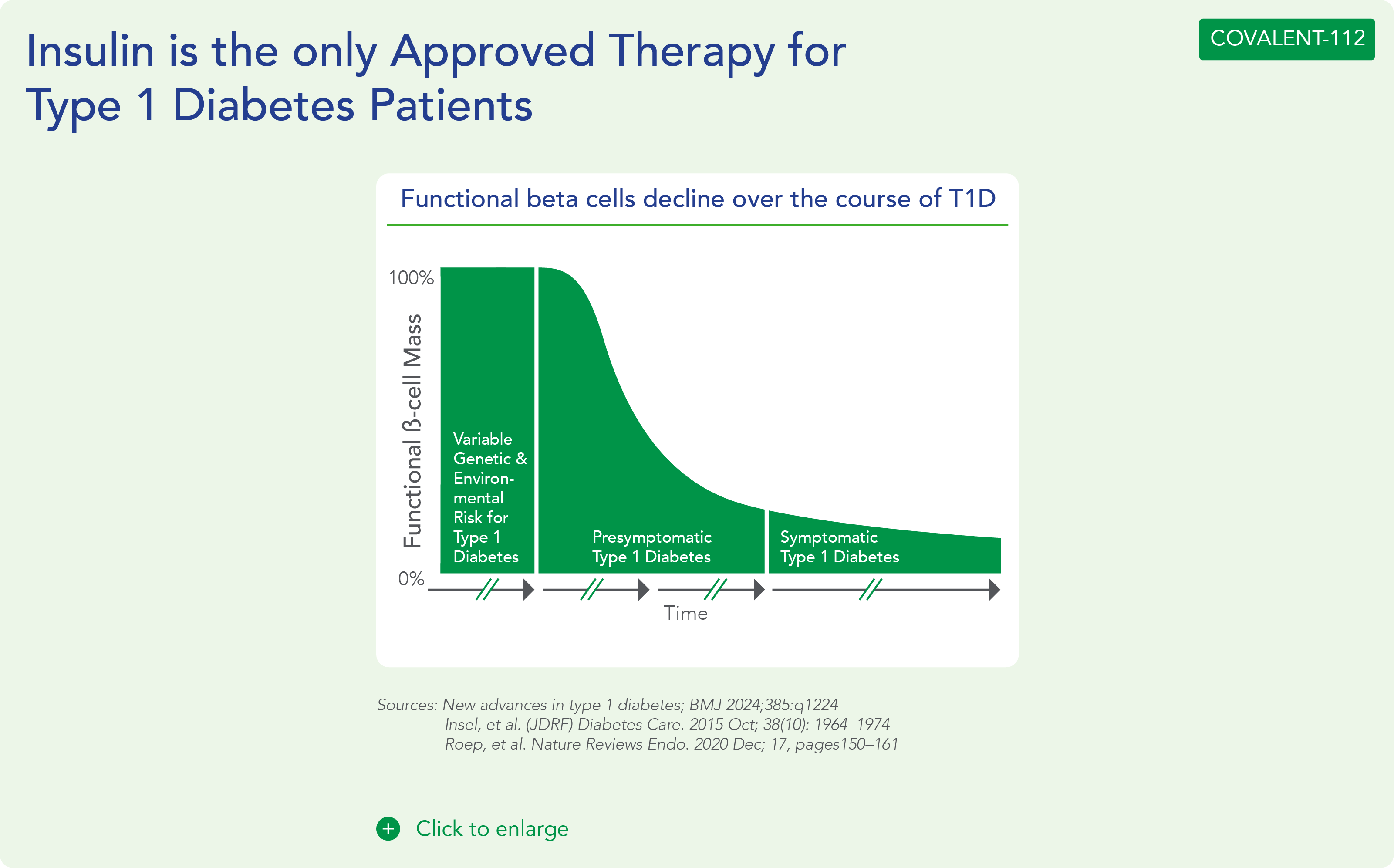
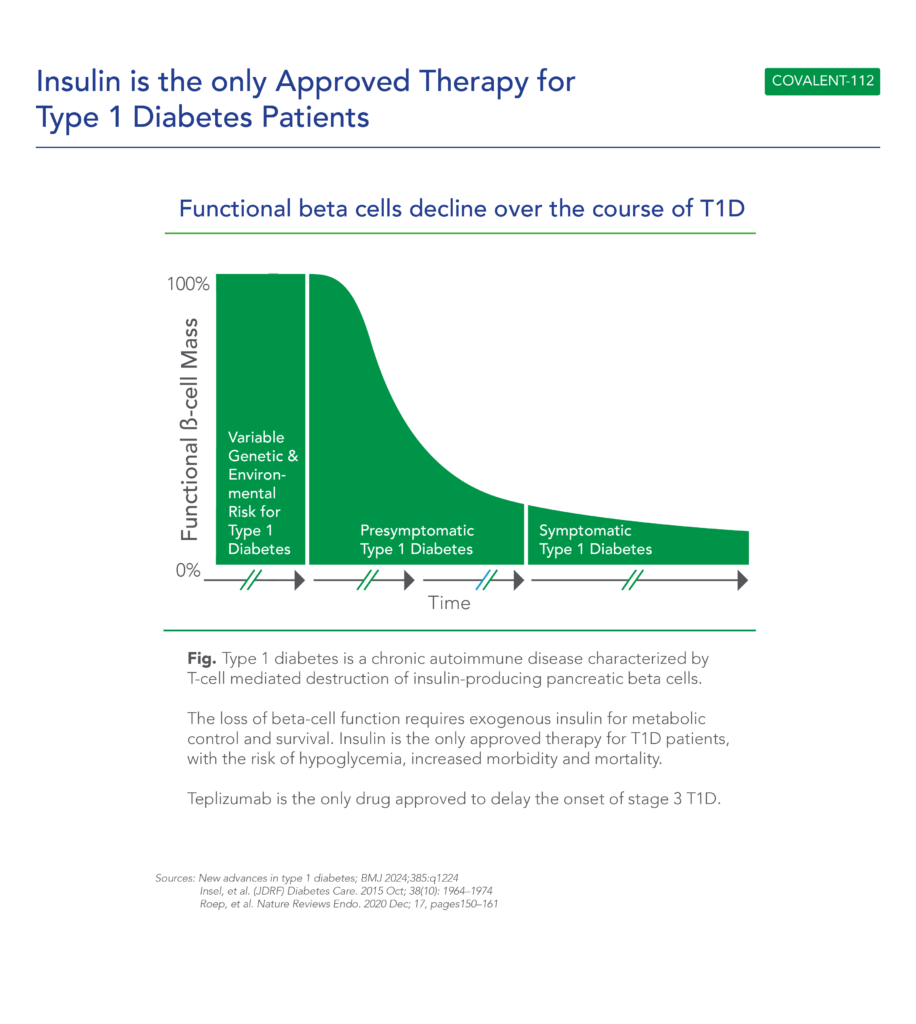
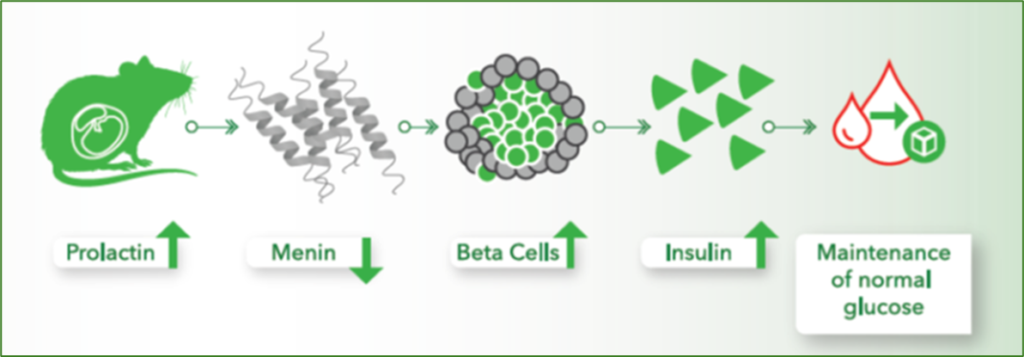
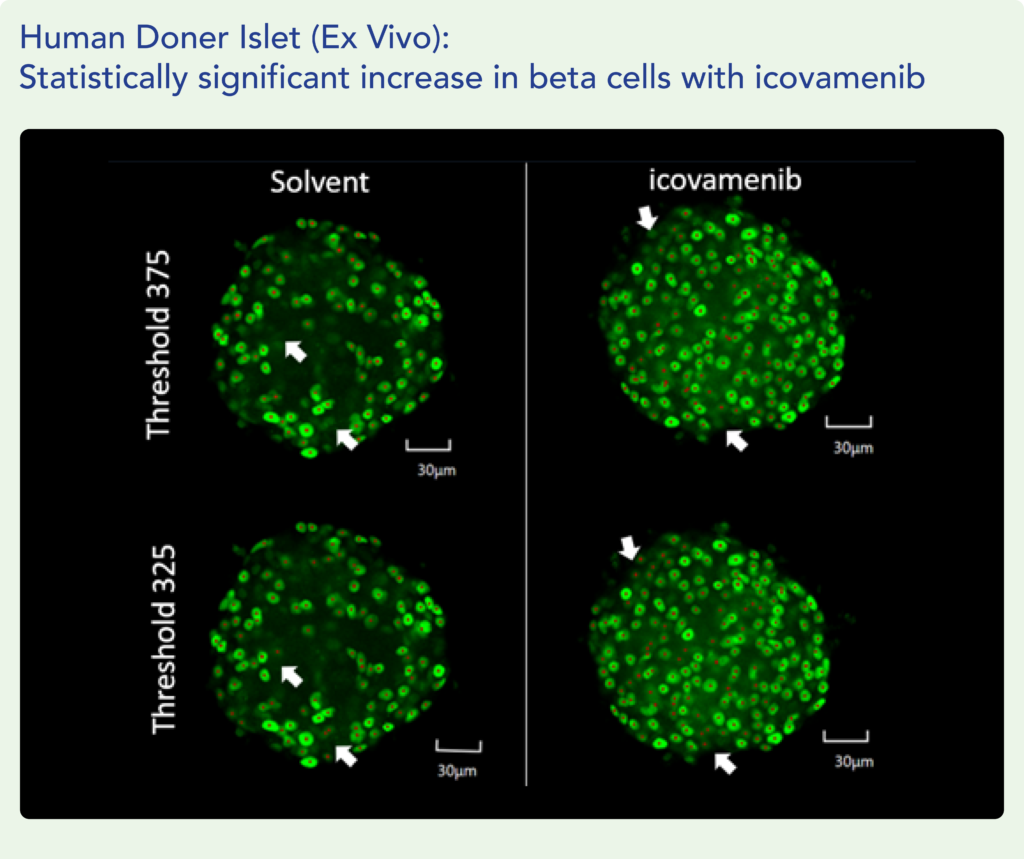

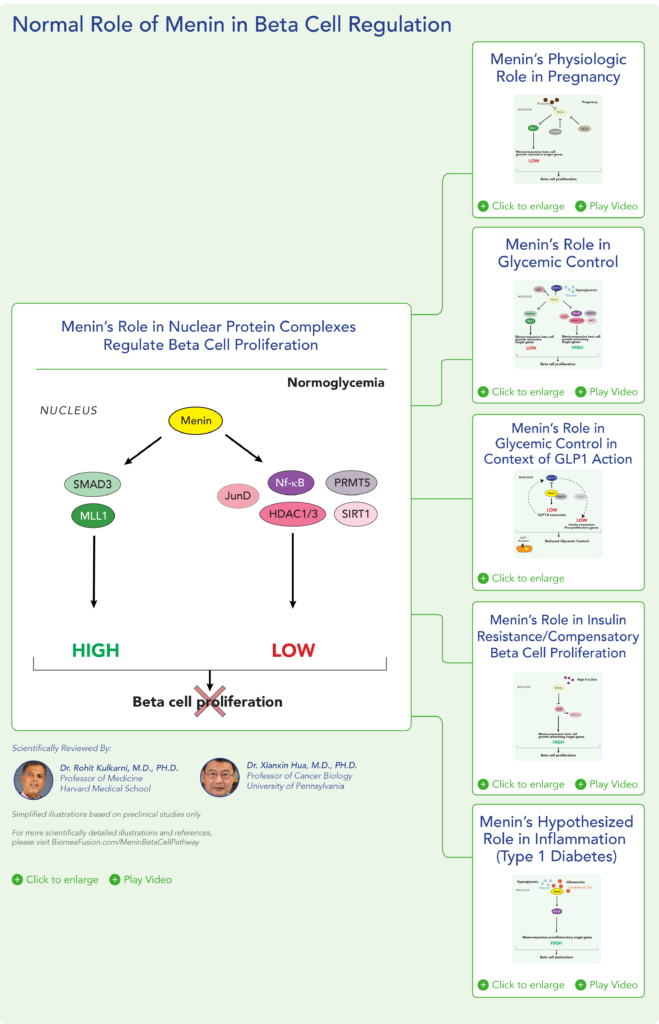
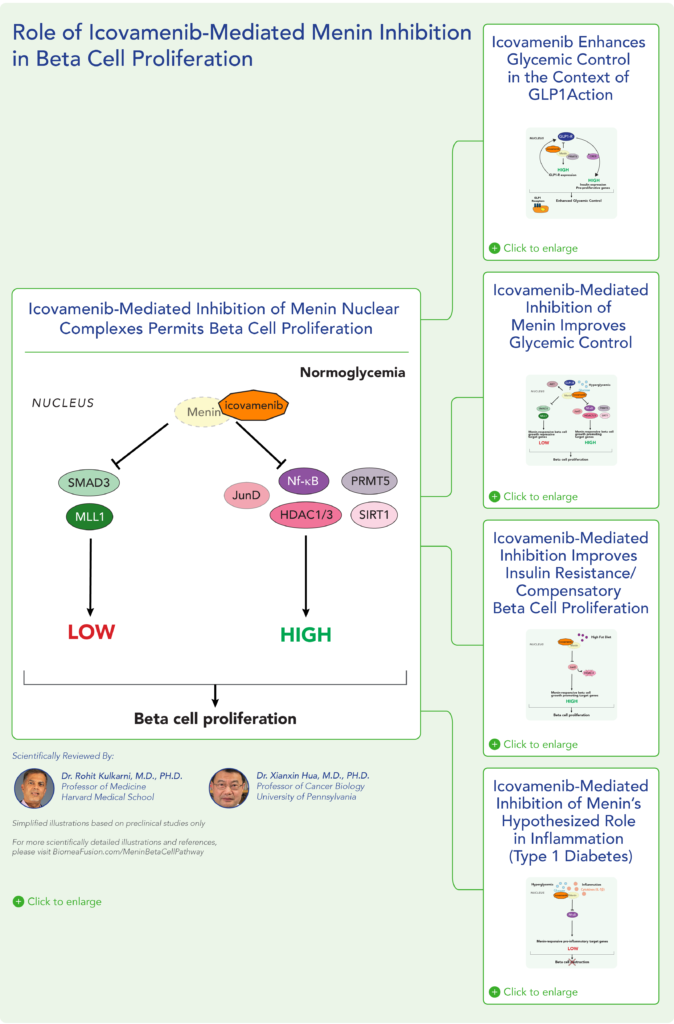


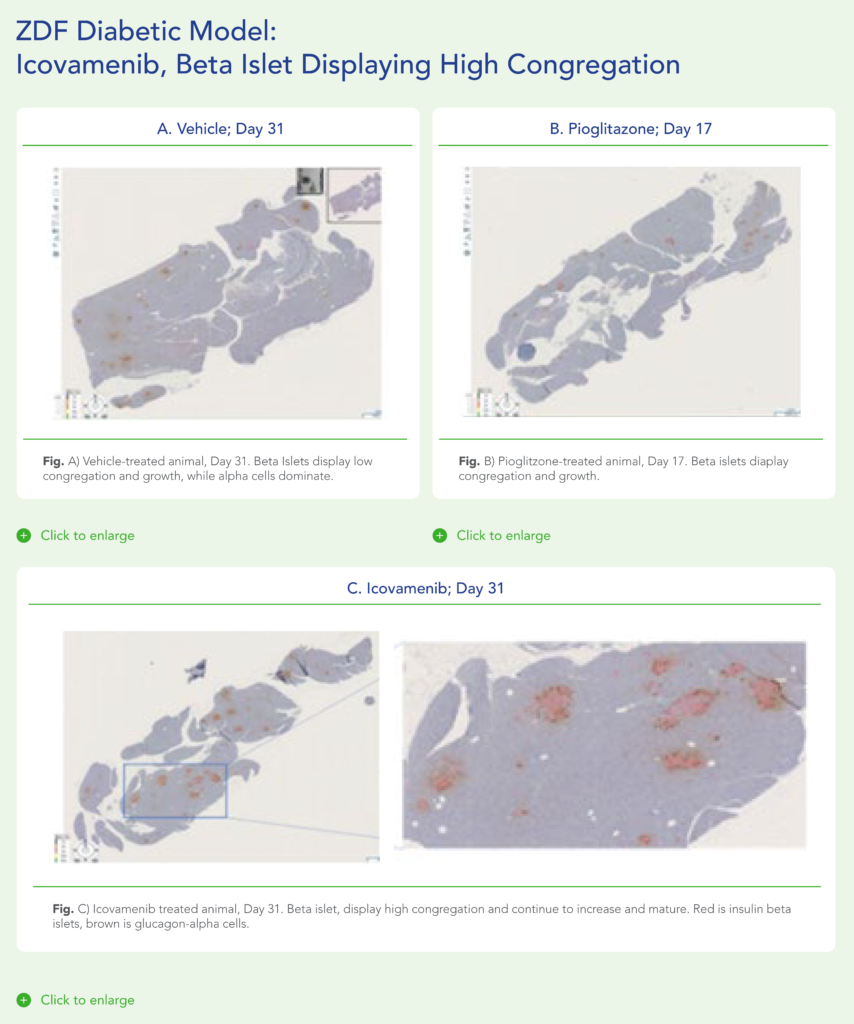
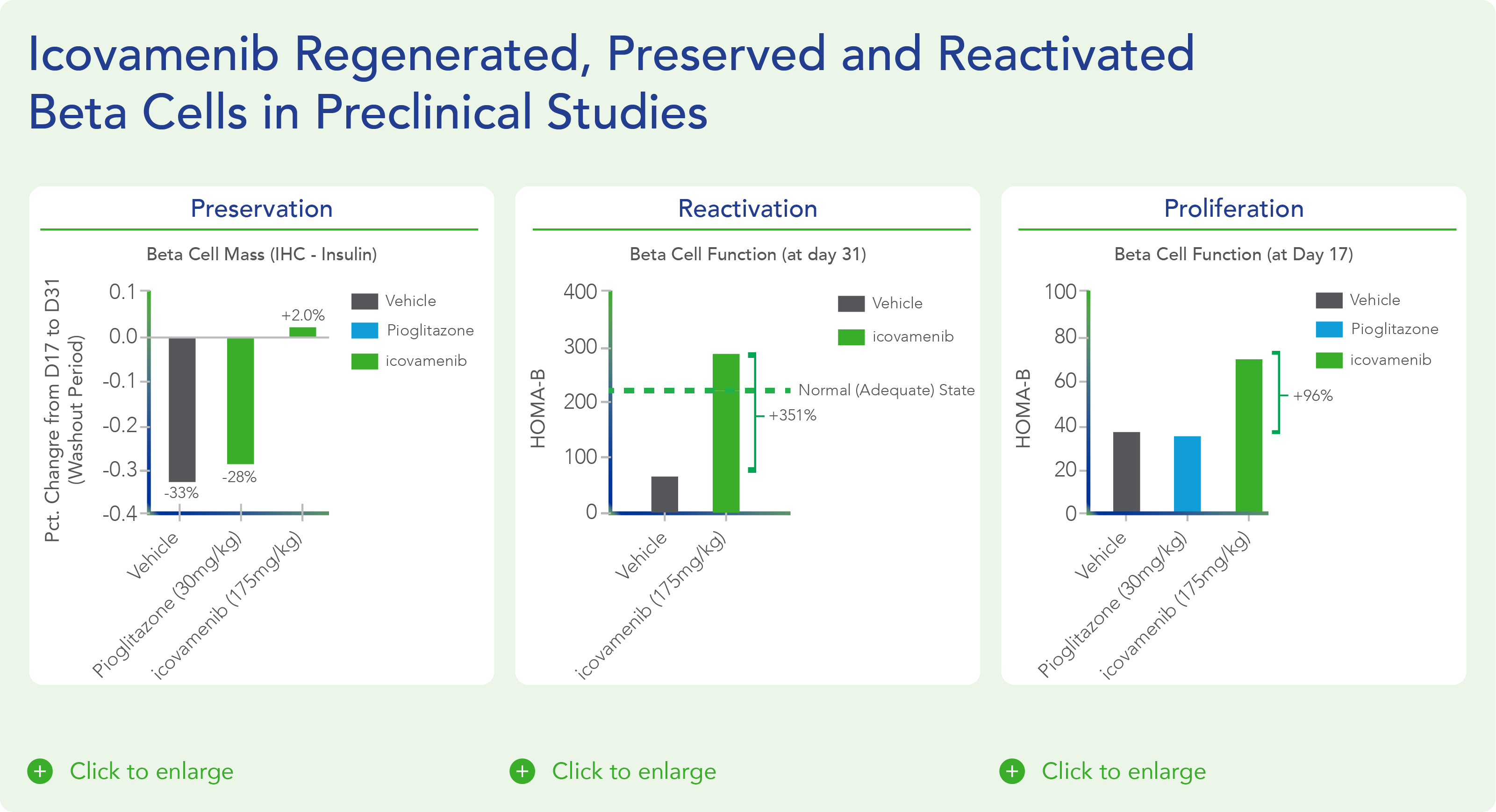
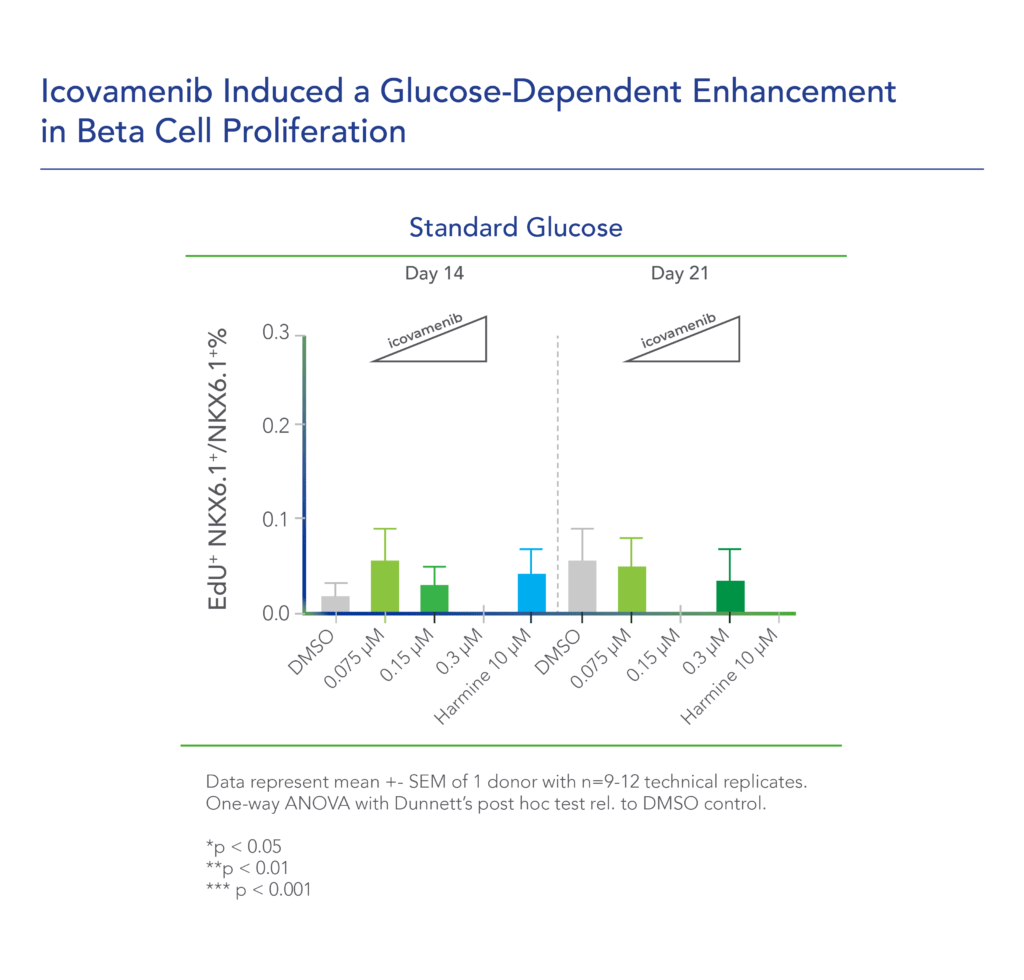

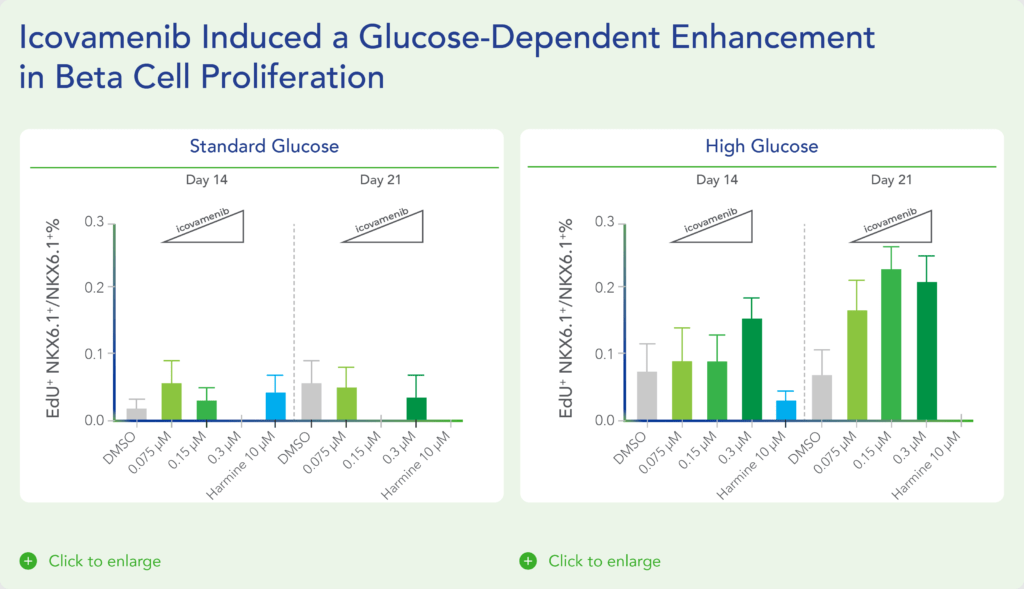






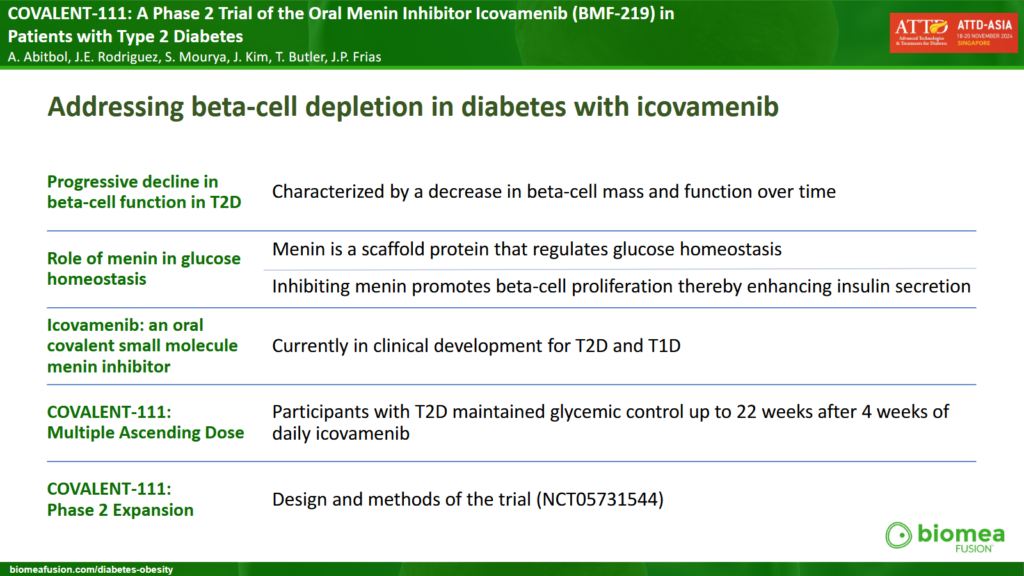
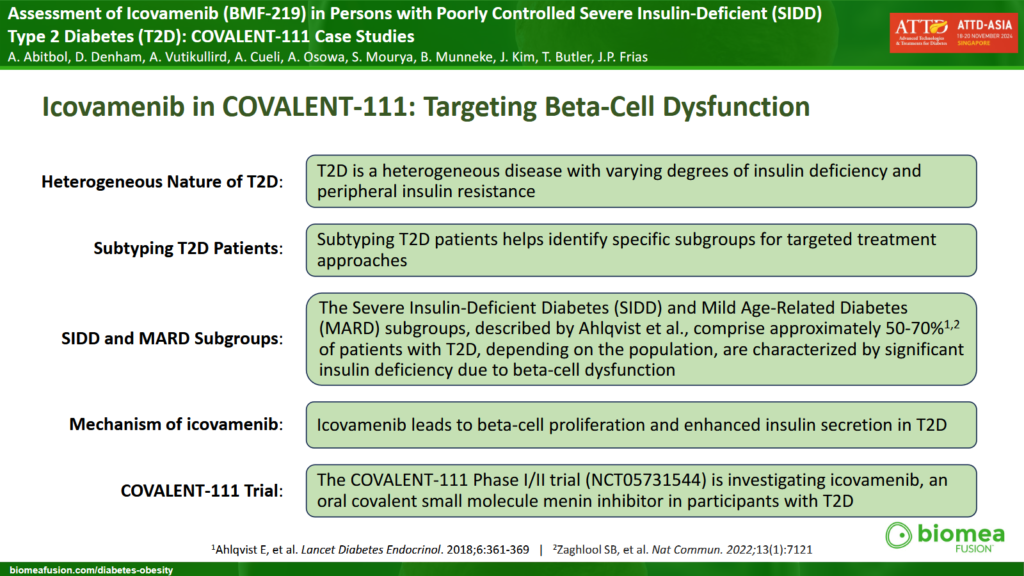




















![Literature References Covalent Inhibition Diabetes and Beta Cell Function Beta Cell Proliferation Menin and Beta Cell Proliferation Type 2 Diabetes Subgroup Analysis Menin in Hematological Malignancies Biomea Publications: Diabetes Biomea Publications: Oncology Covalent Inhibition The article discusses recent innovations in covalent drug discovery, covering various aspects such as the design principles, mechanisms of action, and applications of covalent drugs. It emphasized how advancements in chemical biology and medicinal chemistry have enabled the development of covalent drugs that target specific disease mechanisms with enhanced potency and selectivity. The review also highlights the potential of covalent drugs in treating challenging diseases in the future. The article explores the emergence and increasing importance of targeted covalent inhibitors in drug discovery. It discusses how these inhibitors are designed to form durable bonds with specific target proteins, leading to enhanced potency and selectivity compared to traditional non-covalent inhibitors. The review covers key principles in the design and optimization of targeted covalent inhibitors, as well as their applications across various disease areas. The article addresses the challenges associated with targeting PPIs, which are crucial for cellular signaling and often implicated in cancer progression. It discusses various approaches and techniques employed in the design of covalent PPI inhibitors, emphasizing the need for specificity and potency. Case studies and examples are provided in preclinical and clinical settings, highlighting their potential in overcoming resistance mechanisms and enhancing treatment efficacy in cancer. This perspective article discusses fundamental concepts such as the mechanisms of covalent binding between drugs and their targets, as well as factors influencing the kinetics of these interactions. The author explores how understanding the kinetic properties of covalent and irreversible inhibitors can inform drug design and optimization processes. The article also addresses the implications of kinetic parameters on efficacy, selectivity, and safety profiles of covalent drugs. The article discusses the advantages of covalent drugs regarding enhancing potency and selectivity. It highlights the challenges in designing covalent drugs, such as ensuring specificity and minimizing off-target effects to minimize potential toxicity. The review also covers various strategies for optimizing covalent drug candidates, including medicinal chemistry approaches and the use of advanced screening techniques. [back to top] Diabetes and Beta Cell Function Type 2 diabetes-a matter of beta-cell life and death? Christopher J. Rhodes Science – 2005 Jan 21, 307(5708):380-4. doi: 10.1126/science.1104345. PMID: 15662003. https://pubmed.ncbi.nlm.nih.gov/15662003/ The article explores the pivotal role of beta-cell dysfunction and death in the pathophysiology of type 2 diabetes mellitus (T2DM). It discusses how the progressive loss of beta-cell function and mass contributes to the inability to maintain normal blood glucose levels in individuals with T2DM, highlighting factors such as genetic predisposition, lifestyle choices, and environmental factors that influence beta-cell health and function. The review suggests that strategies aimed at preserving beta-cell mass and function could hold promise for preventing and managing T2DM. Diabetes Invest_Beta-cell failure in diabetes – Common susceptibility and mechanisms shared between type 1 and type 2 diabetes 1 Hiroshi Ikegami, Naru Babaya, and Shinsuke Noso Journal of Diabetes Investigation – 2021 Sep; 12(9): 1526–1539. Published online 2021 Jun 16. doi: 10.1111/jdi.13576 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8409822/ The article explores the shared susceptibility and underlying mechanisms of beta-cell dysfunction in both T1DM and T2DM. It discusses how genetic predisposition, autoimmune responses, and environmental factors contribute to the loss of beta-cell function in both forms of diabetes. The review emphasizes future research directions aimed at addressing beta-cell failure as a key strategy in managing and potentially preventing both T1DM and T2DM. Not control but conquest – Strategies for the remission of Type 2 diabetes mellitus Jinyoung Kim, Hyuk-Sang Kwon Diabetes and Metabolism Journal – 2022 Mar;46(2):165-180. doi: 10.4093/dmj.2021.0377. https://pubmed.ncbi.nlm.nih.gov/35385632/ The article explores strategies aimed at achieving remission rather than mere control of T2DM. It discusses various approaches including lifestyle modifications, pharmacotherapy, and bariatric surgery, which have shown potential in achieving sustained remission of T2DM. It also emphasizes personalized treatment plans tailored to individual patient characteristics such as age, duration of diabetes, and comorbidities. The review also covers emerging therapies such as GLP-1 receptor agonists and SGLT-2 inhibitors, which have demonstrated efficacy in improving beta-cell function and insulin sensitivity. Increased beta-cell proliferation before immune cell invasion prevents progression of Type 1 diabetes Dirice E et al., 2019 Nature Metabolism Nat Metab – 2019 May; 1(5): 509–518. Published online 2019 May 6. doi: 10.1038/s42255-019-0061-8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6696912/ The study explores how enhanced beta-cell replication prior to immune cell infiltration can mitigate the development of T1D. The researchers utilize mouse models and human pancreatic tissue to demonstrate that beta-cells exhibit increased proliferation in response to specific conditions, which leads to improved glucose tolerance and delays in T1D onset. The study underscores the potential therapeutic implications of promoting beta-cell proliferation as a strategy to prevent or delay the progression of T1D. Importance of beta cell mass for glycaemic control in people with Type 1 diabetes Theodorus J P Jansen, et. al., Diabetologia, 2023 Feb; 66(2):367-375. doi: 10.1007/s00125-022-05830-2. Epub 2022 Nov 17. https://pubmed.ncbi.nlm.nih.gov/36394644/ The article discusses how the preservation or restoration of beta-cell mass is crucial for achieving stable blood glucose levels and minimizing complications associated with T1D. It reviewed current research and clinical evidence highlighting that even small residual amounts of beta-cell function can significantly improve glycemic outcomes and reduce the risk of hypoglycemia. Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes Yoshifumi Saisho International Journal of Molecular Science – 2016 May 17; 17(5):744. doi: 10.3390/ijms17050744. PMID: 27196896; PMCID: PMC4881566. https://pubmed.ncbi.nlm.nih.gov/27196896/ The article examines the utility of the postprandial C-peptide to glucose ratio as an indicator of beta-cell function in individuals with T2D and discusses how this ratio reflects the insulin secretion relative to glucose levels after meals, providing insights into beta-cell health and function beyond fasting measurements. The review suggests that monitoring postprandial C-peptide to glucose ratios could improve the management of T2D by assessing beta-cell responsiveness and predicting treatment outcomes as a valuable tool in personalized diabetes care. Remission of human Type 2 diabetes requires decrease on liver and pancreas fat content, but is dependent upon capacity for beta cell recovery Taylor R et al. Cell Metabolism, 2018 Oct 2; 28(4):547-556.e3. doi: 10.1016/j.cmet.2018.07.003. https://pubmed.ncbi.nlm.nih.gov/30078554/ The study explores how reducing fat accumulation in the liver and pancreas is critical for T2D remission. It highlights that while weight loss and fat reduction are crucial, sustained remission also depends on the capacity of beta cells to recover and improve insulin secretion. The findings underscore the complex interplay between metabolic factors and beta-cell health in achieving and maintaining T2D remission, suggesting comprehensive approaches that address both fat accumulation and beta-cell recovery are essential for long-term management of the disease. Intervention with therapeutic agents, Understanding the path to remission in Type 2 Diabetes: Part 1](https://biomeafusion.com/wp-content/uploads/2024/08/Diabetes-and-Beta-Cell-Function_BC_Blue-08-791x1024.png)